3.6: Resonance - Equivalent Lewis Structures for the Same Molecule
- Page ID
- 357385
Resonance
There are some cases in which more than one viable Lewis structure can be drawn for a molecule. An example is the ozone, \(\left( \ce{O_3} \right)\), a greenhouse gas and contributes to climate change, molecule in Figure \(\PageIndex{1}\). There are a total of 18 electrons in the structure and so the following two structures are possible.
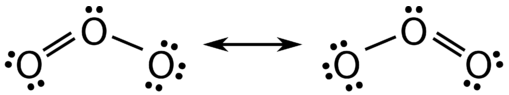
The structure on the left (\(\PageIndex{1}\)) can be converted to the structure on the right by a shifting of electrons without altering the positions of the atoms.
It was once thought that the structure of a molecule such as \(\ce{O_3}\) consisted of one single bond and one double bond which then shifted back and forth as shown above. However, further studies showed that the two bonds are identical. Any double covalent bond between two given atoms is typically shorter than a single covalent bond. Studies of the \(\ce{O_3}\) and other similar molecules showed that the bonds were identical in length. Interestingly, the length of the bond is in between the lengths expected for an \(\ce{O-O}\) single bond and a double bond.
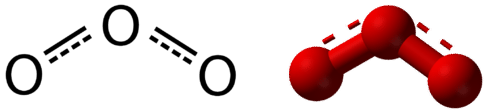
Resonance is the use of two or more Lewis structures to represent the covalent bonding in a molecule. One of the valid structures is referred to as a resonance structure. It is now understood that the true structure of a molecule which displays resonance is that of an average or a hybrid of all the resonance structures. In the case of the \(\ce{O_3}\) molecule, each of the covalent bonds between \(\ce{O}\) atoms is best thought of as being "one and a half" bonds, as opposed to either a pure single bond or a pure double bond. This "half-bond" can be shown as a dotted line in both the Lewis structure and the molecular model (Figure \(\PageIndex{2}\)).
Summary
-
It is possible to draw resonance structures for molecules that are not viable structures. Additional guidelines for assessing the validity of a Lewis structure beyond the octet rule (or duet for hydrogen) are beyond the scope of this course.
-
The resonance structures only differ in the placement of electrons – the connectivity of the atoms in the molecule remains the same between resonance structures.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)

