14.9.1: Nuclear Fission
- Page ID
- 478418
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the process of nuclear fission.
Nuclear fission was first discovered by Lise Meitner and Otto Hahn, in the 1930s. They began their work by bombarding uranium with neutrons, hoping to create larger elements. Instead, they were very surprised to find \(\ce{Ba}\)-141, a much smaller element. It was many years before Lise's contribution to this discovery was fully understood, in part because she had to complete much of her work as a refugee who had to flee Germany during World War II. Hahn was later a sole recipient of a Nobel Prize for this work. Meitner was critical of Hahn for not giving her credit for her work, not speaking out against the Nazi regime, and critical of her colleagues in nuclear physics for the use of this research to create the atomic bomb.
Nuclear Fission
Radioactive decay by the release of alpha or beta particles is not the only way new isotopes are formed. When a neutron collides with a nucleus, the nucleus splits into two isotopes, each of which is roughly half the mass of the original atom. A small amount of mass is "left over" and released as energy, as predicted by Einstein's famous equation \(E = mc^2\), that relates mass and energy. This process is known as nuclear fission. The neutron must be a "slow" neutron, traveling at a speed that is approximately that of the molecules of a gas at the same temperature in the system producing the neutrons. High-speed ("fast") neutrons will not result in nuclear fission.
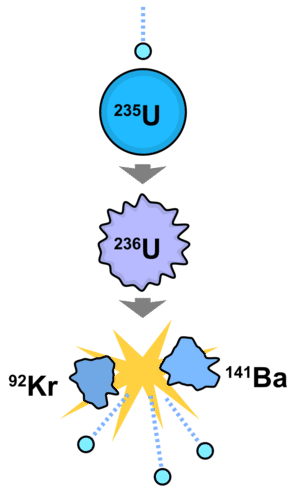
The example above illustrates the basic nuclear fission process. A neutron (generally produced by some controlled process, not usually a natural event) collides with an atom of \(\ce{U}\)-235. Momentarily, a \(\ce{U}\)-236 atom forms, which then splits into two smaller atoms (\(\ce{Kr}\)-93 and \(\ce{Ba}\)-141) in the diagram. This process results in the release of three new neutrons, which can then initiate fission reactions with more atoms. We will see later how this propagation of neutrons can be employed in a reactor for the generation of electricity.
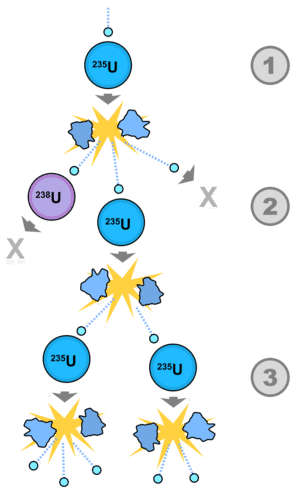
An extended version of this process can be seen in the figure below. Not every collision of a neutron with \(\ce{U}\)-235 results in a fission reaction. A neutron from the initial fission process may strike an atom of \(\ce{U}\)-238, which does not continue the process. Another neutron may not collide with a nucleus, and be lost in the environment. However, a third neutron produced from the initial collision can collide with more \(\ce{U}\)-235, and continue the chain reaction to produce more neutrons.
Typical nuclear fission reactions balance in terms of mass. The total mass of the reactants is equal to the total mass of the products:
\[\ce{^{235}_{92}U} + \ce{^1_0n} \rightarrow \ce{^{92}_{36}Kr} + \ce{^{142}_{56}Ba} + 2 \ce{^1_0n} + \text{energy}\nonumber \]
There are a total of 236 mass units on the left of the equation and 236 mass units on the right. In the same manner, we see 92 protons on the left and 92 on the right. The energy that is released is the binding energy that holds the nucleus together.
Another set of fission products from \(\ce{U}\)-235 can be seen in the following reaction:
\[\ce{^{235}_{92}U} + \ce{^1_0n} \rightarrow \ce{^{95}_{42}Mo} + \ce{^{139}_{57}La} + 2 \ce{^1_0n} + \text{energy}\nonumber \]
Again, we see that the total number of mass units and of protons is equal on both sides of the equation.
Nuclear Power Generation
The generation of electricity is critical for operation of businesses, health care delivery, schools, homes, and other areas requiring the use of electrical power. According to 2011 statistics, coal is used for \(42\%\) of the total power generated, with natural gas being employed for another \(25\%\). Nuclear power plants are employed in about \(19\%\) of the cases, with renewable energy sources supplying the last \(13\%\). All of these fuels are used to heat water to generate steam. The steam then turns a turbine to generate electricity.
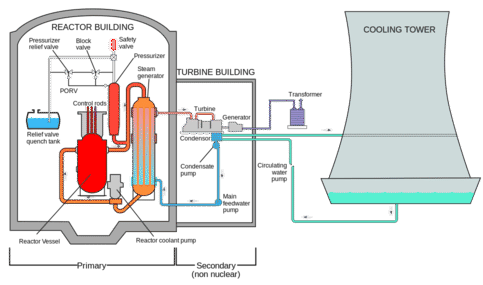
The diagram below shows the layout of a typical nuclear power plant. The radioactive rods are in the red container along with water, which is heated to steam. The energy for this heat comes from fission reactions of uranium. The steam passes through the turbine and causes the turbine to spin, generating electricity. As the steam condenses, it is run through a cooling tower to lower its temperature. The water then recirculates through the reactor core to be used again.
The control rods play an important role in the modulation of the nuclear chain reaction (usually a collision of a neutron with uranium). Each collision produces more neutrons than were present initially. If left unsupervised, the reaction would soon get out of control. Rods are commonly made of boron or a number of metals and metal alloys. The purpose of the control rods is to absorb neutrons to regulate the rate of the chain reaction, so that the water does not overheat and destroy the reactor.
Nuclear power is also used to propel ships. The turbine can be connected to a propeller system. The rotating turbine shaft will turn the propeller to move the ship.

Section Summary
- Nuclear fission is the process of a neutron colliding with a nucleus. The nucleus splits into two isotopes, each of which is roughly half the mass of the original atom. A small amount of mass is "left over" and released as energy.
- Examples of nuclear fission processes are illustrated.
- The importance of nuclear power in generating electricity is described.
- The operation of a nuclear power plant is described.


