13.8: Acid Base Reactions
- Page ID
- 476649
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify acids and bases both in terms of their characteristic properties and in terms of their chemical reactivity.
- Identify acidic or basic solutions using the pH scale.
You are probably familiar with the terms acid, base, and pH. It turns out that acids and bases show up in our daily lives all the time: the foods we eat, the cleaning supplies we use, and even breathing involve acid base chemistry. In the section we will discuss these concepts in more detail as we explore acid base reactions.
Acids
Acids are very common in some of the foods that we eat. Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid, which is better known as vitamin C. Carbonated sodas contain phosphoric acid. Vinegar contains acetic acid. Your own stomach utilizes hydrochloric acid to digest food.
Acids are a distinct class of compounds because of the properties of their aqueous solutions. These properties are:
- Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Other acids are weak electrolytes that exist primarily in a non-ionized form when dissolved in water.
- Acids have a sour taste. Lemons, vinegar, and sour candies all contain acids.
- Acids change the color of certain acid-base indicates. Two common indicators are litmus and phenolphthalein. Blue litmus turns red in the presence of an acid, while phenolphthalein turns colorless.
- Acids react with active metals to yield hydrogen gas. Recall that an activity series is a list of metals in descending order of reactivity. Metals that are above hydrogen in the activity series will replace the hydrogen from an acid in a single-replacement reaction, as shown below:
\[\ce{Zn} \left( s \right) + \ce{H_2SO_4} \left( aq \right) \rightarrow \ce{ZnSO_4} \left( aq \right) + \ce{H_2} \left( g \right)\nonumber \] - Acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. The products of this reaction are an ionic compound, which is labeled as a salt, and water.
Bases
Bases have properties that mostly contrast with those of acids.
- Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just as acids can.
- Bases often have a bitter taste and are found in foods less frequently than acids. Many bases, like soaps, are slippery to the touch.
- Bases also change the color of indicators. Litmus turns blue in the presence of a base while phenolphthalein turns pink.
- Bases do not react with metals in the way that acids do.
- Bases react with acids to produce a salt and water.
Figure \(\PageIndex{1}\): Phenolphthalein indicator in presence of base.
Please note that tasting chemicals and touching them are NOT good lab practices and should be avoided—in other words, do not do this at home.
Bases are less common as foods, but they are nonetheless present in many household products. Many cleaners contain ammonia, a base. Sodium hydroxide is found in drain cleaner. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium hydrogen carbonate.
Arrhenius Acids and Bases
Swedish chemist Svante Arrhenius (1859-1927) was the first to propose a theory to explain the observed behavior of acids and bases. Because of their ability to conduct a current, he knew that both acids and bases contained ions in solution. An Arrhenius acid is a compound that ionizes to yield hydrogen ions \(\left( \ce{H^+} \right)\) in aqueous solution. An Arrhenius base is a compound that ionizes to yield hydroxide ions \(\left( \ce{OH^-} \right)\) in aqueous solution.
Acids are molecular compounds with ionizable hydrogen atoms. Only hydrogen atoms that are part of a highly polar covalent bond are ionizable. Hydrogen chloride \(\left( \ce{HCl} \right)\) is a gas at room temperature and under normal pressure. The \(\ce{H-Cl}\) bond in hydrogen chloride is a polar bond. The hydrogen atom is electron deficient because of the higher electronegativity of the chlorine atom. Consequently, the hydrogen atom is attracted to the lone pair of electrons in a water molecule when \(\ce{HCl}\) is dissolved in water. The result is that the \(\ce{H-Cl}\) bond breaks, with both bonding electrons remaining with the \(\ce{Cl}\), forming a chloride ion. The \(\ce{H^+}\) ion attaches to the water molecule, forming a polyatomic ion called the hydronium ion. The hydronium ion \(\left( \ce{H_3O^+} \right)\) can be thought of as a water molecule with an extra attached hydrogen ion.
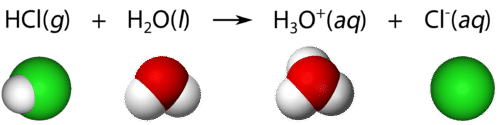
Equations showing the ionization of an acid in water are frequently simplified by omitting the water molecule:
\[\ce{HCl} \left( g \right) \rightarrow \ce{H^+} \left( aq \right) + \ce{Cl^-} \left( aq \right)\nonumber \]
This is merely a simplification of the previous equation, but it is commonly used. Any hydrogen ions in an aqueous solution will be attached to water molecules as hydronium ions.
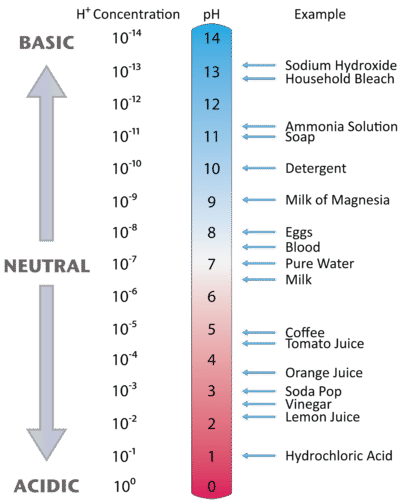
The pH Scale
Expressing the acidity of a solution by using the molarity of the hydrogen ion is cumbersome because the quantities are generally very small. Danish scientist Søren Sørensen (1868-1939) proposed an easier system for indicating the concentration of \(\ce{H^+}\) called the pH scale. The letters pH stand for the power of the hydrogen ion. The pH of a solution is the negative logarithm of the hydrogen-ion concentration:
\[\text{pH} = -\text{log} \: \left[ \ce{H^+} \right]\nonumber \]
In pure water or a neutral solution, the \(\left[ \ce{H^+} \right] = 1.0 \times 10^{-7} \: \text{M}\). Substituting into the pH expression:
\[\text{pH} = -\text{log} \left[ 1.0 \times 10^{-7} \right] = -\left( -7.00 \right) = 7.00\nonumber \]
The pH of pure water or any neutral solution is thus 7.00. For recording purposes, the numbers to the right of the decimal point in the pH value are the significant figures. Since \(1.0 \times 10^{-7}\) has two significant figures, the pH can be reported as 7.00.
A logarithmic scale condenses the range of acidity to numbers that are easy to use. Consider a solution with \(\left[ \ce{H^+} \right] = 1.0 \times 10^{-4} \: \text{M}\). That is a hydrogen-ion concentration that is 1000 times higher than the concentration in pure water. The pH of such a solution is 4.00, a difference of just 3 pH units. Notice that when the \(\left[ \ce{H^+} \right]\) is written in scientific notation and the coefficient is 1, the pH is simply the exponent with the sign changed. The pH of a solution with \(\left[ \ce{H^+} \right] = 1 \times 10^{-2} \: \text{M}\) is 2 and the pH of a solution with \(\left[ \ce{H^+} \right] = 1 \times 10^{-10} \: \text{M}\) is 10.
As we saw earlier, a solution with \(\left[ \ce{H^+} \right]\) higher than \(1.0 \times 10^{-7}\) is acidic, while a solution with \(\left[ \ce{H^+} \right]\) lower than \(1.0 \times 10^{-7}\) is basic. Consequently, solutions with a pH of less than 7 are acidic, while those with a pH higher than 7 are basic. Figure \(\PageIndex{1}\) illustrates this relationship, along with some examples of various solutions.
Section Summary
- Characteristic properties of acids and bases are provided.
- An Arrhenius acid is a compound that ionizes to yield hydrogen ions \(\left( \ce{H^+} \right)\) in aqueous solution.
- An Arrhenius base is a compound that ionizes to yield hydroxide ions (OH−)(OH−) in aqueous solution.
- The pH of a solution is the negative logarithm of the hydrogen-ion concentration.
- Solutions with a pH of less than 7 are acidic, while those with a pH higher than 7 are basic.
- pH values for several common materials are listed.
Glossary
- Arrhenius acid
- A compound that ionizes to yield hydrogen ions \(\left( \ce{H^+} \right)\) in aqueous solution.
- Arrhenius base
- A compound that ionizes to yield hydroxide ions \(\left( \ce{OH^-} \right)\) in aqueous solution.
- hydronium ion \(\left( \ce{H_3O^+} \right)\)
- A water molecule with an extra hydrogen ion attached to it.
- pH
- The negative logarithm of the hydrogen-ion concentration.



