11.7: Covalent Bonds and Molecules
- Page ID
- 476586
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how covalent bonds form by sharing electrons.
- Describe the characteristic properties of molecules.
In a tennis match, two players keep hitting the ball back and forth. The ball bounces from one player to the other, over and over again. The ball keeps the players moving together on the court. What if the two players represented the nuclei of two atoms and the ball represented valence electrons? What would the back and forth movement of the ball represent? The answer is a covalent bond.
Covalent Bonds: Sharing Electrons
A covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. The shared electrons are attracted to the nuclei of both atoms. This forms a molecule consisting of two or more atoms. Covalent bonds form only between atoms of nonmetals.
The two atoms that are held together by a covalent bond may be atoms of the same element or different elements. When atoms of different elements form covalent bonds, a new substance, called a covalent compound, results. All covalent compounds are also molecules. Water is an example of a covalent compound. A water molecule is modeled in the figure below. A molecule is the smallest particle of a covalent compound that still has the properties of the compound.
How many valence electrons does the oxygen atom (O) share with each hydrogen atom (H)? How many covalent bonds hold the water molecule together? (Refer to Figure \(\PageIndex{2}\))
- Answer
-
The oxygen atom shares one pair of valence electrons with each hydrogen atom. Each pair of shared electrons represents one covalent bond, so two covalent bonds hold the water molecule together.
The diagram in the figure below shows an example of covalent bonds between two atoms of the same element, in this case two atoms of oxygen. The diagram represents an oxygen molecule, so it’s not a new compound. Oxygen normally occurs in diatomic (“two-atom”) molecules. Several other elements also occur as diatomic molecules: hydrogen, nitrogen, and all but one of the halogens (fluorine, chlorine, bromine, and iodine).
How many electrons do these two oxygen atoms share? How many covalent bonds hold the oxygen molecule together?(Refer to Figure \(\PageIndex{3}\))
- Answer
-
The two oxygen atoms share two pairs of electrons, so two covalent bonds hold the oxygen molecule together.
Why Covalent Bonds Form
Covalent bonds form because they give atoms a more stable arrangement of electrons. Nonmetals in molecules follow the Octet Rule by sharing electrons in the formation of a covalent bond in such a way that each of the atoms involved in the bond can attain a noble-gas electron configuration. The shared electrons are "counted" for each of the atoms involved in the sharing. For hydrogen (H2)(H2), the shared pair of electrons means that each of the atoms is able to attain the electron configuration of helium, the noble gas with two electrons. For atoms other than hydrogen, the sharing of electrons will usually provide each of the atoms with eight valence electrons
Multiple Covalent Bonds
Some molecules are not able to satisfy the octet rule by making only single covalent bonds between the atoms. Consider the compound ethene, which has a molecular formula of \(\ce{C_2H_4}\). The carbon atoms are bonded together, with each carbon also bonded to two hydrogen atoms.
two \(\ce{C}\) atoms \(= 2 \times 4 = 8\) valence electrons
four \(\ce{H}\) atoms \(= 4 \times 1 = 4\) valence electrons
= total of 12 valence electrons in the molecule
If the Lewis electron dot structure was drawn with a single bond between the carbon atoms and with the octet rule followed, it would look like this:
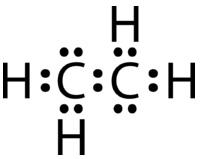
This Lewis structure is incorrect because it contains a total of 14 electrons. However, the Lewis structure can be changed by eliminating the lone pairs on the carbon atoms and having the carbon atoms share two pairs, instead of only one pair, of electrons.
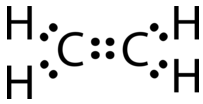
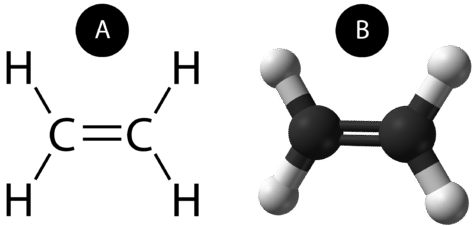
A double covalent bond is a covalent bond formed by atoms that share two pairs of electrons. The double covalent bond that occurs between the two carbon atoms in ethane can also be represented by a structural formula and with a molecular model, as shown in the figure below.
A triple covalent bond is a covalent bond formed by atoms that share three pairs of electrons. The element nitrogen is a gas that composes the majority of Earth's atmosphere. A nitrogen atom has five valence electrons, which can be shown as one pair and three single electrons. When combining with another nitrogen atom to form a diatomic molecule, the three single electrons on each atom combine to form three shared pairs of electrons.
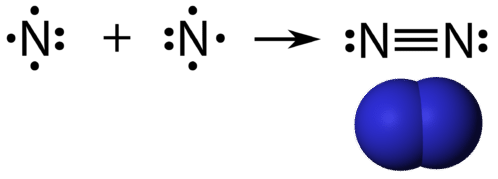
Each nitrogen atom follows the octet rule with one lone pair of electrons, and six electrons that are shared between the atoms.
Properties of Molecules
The covalent bonds of molecules are responsible for many of their properties, regardless of whether they are elements or compounds. Because valence electrons are shared in molecules, rather than transferred between atoms as they are in ionic compounds, molecules have very different properties than ionic compounds.
- Many covalent compounds, especially those containing carbon and hydrogen, burn easily. In contrast, many ionic compounds do not burn.
- Many covalent compounds do not dissolve in water, whereas most ionic compounds dissolve well in water.
- Unlike ionic compounds, covalent compounds do not have freely moving electrons, so they cannot conduct electricity.
- The individual molecules of covalent compounds are more easily separated than the ions in a crystal, so most covalent compounds have relatively low boiling points. This explains why many of them are liquids or gases at room temperature.
Section Summary
- A covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. Covalent bonds form only between atoms of nonmetals.
- The two atoms that are held together in a covalent bond may be atoms of the same element or different elements. When atoms of different elements bond together, it forms a covalent compound.
- Covalent bonds form because the shared electrons fill each atom’s outer energy level and this is the most stable arrangement of electrons.
- Lewis electron-dot structures show the bonding in covalent molecules.
- Covalent compounds contain two or more nonmetallic elements held together by covalent bonds, in which atoms share pairs of valence electrons. A molecule is the smallest particle of a covalent compound that still has the properties of the compound.
- Covalent bonds are responsible for many of the properties of covalent compounds. Covalent compounds have relatively low boiling points, cannot conduct electricity, and may not dissolve in water.
- Covalent bonds between atoms can be indicated either with dots (:) or a dash (−).
- Lewis structures can be drawn for molecules that share multiple pairs of electrons.
- A double covalent bond is a covalent bond formed by atoms that share two pairs of electrons.
- A triple covalent bond is a covalent bond formed by atoms that share three pairs of electrons.


