2.3: Video- Atomic Laws
- Page ID
- 232978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Law of Conservation of Mass
The Law of Conservation of Mass states that matter is not created or destroyed—it only changes form.
Law of Definite Proportions
The Law of Definite Proportions states that a compound will always contain the same ratio (of either atoms or mass) of the same elements.
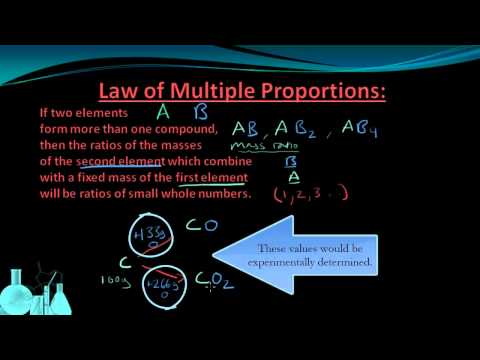
Law of Multiple Proportions
If two elements combine in a different ratio, the result is a different compound. In other words, the same compound will always form from the same elements in the same ratio.
All rights reserved content
- Chemistry Concepts: Conservation of Mass/Energy. Authored by: Thirst for Science. Located at: https://youtu.be/J5hM1DxaPLw. License: All Rights Reserved. License Terms: Standard YouTube license
- Chemistry 5.8a Law of Definite Proportions. Authored by: IsaacsTEACH. Located at: https://youtu.be/nvTB2cMbWU8. License: All Rights Reserved. License Terms: Standard YouTube license
- Chemistry 5.8b Law of Multiple Proportions. Authored by: IsaacsTEACH. Located at: https://youtu.be/l-IPd_r7ytw. License: All Rights Reserved. License Terms: Standard YouTube license