12.1 Oxidation-Reduction Reactions
- Last updated
- Save as PDF
- Page ID
- 171969
Learning Objectives
- Define oxidation and reduction.
- Assign oxidation numbers to atoms in simple compounds.
- Recognize a reaction as an oxidation-reduction reaction.
Consider this chemical reaction:
\[\ce{Mg(s) + Cl2(g) → MgCl2}\]
The reactants are two electrically neutral elements; they have the same number of electrons as protons. The product, however, is ionic; it is composed of Mg2+ and Cl− ions. Somehow, the individual Mg atoms lose two electrons to make the Mg2+ ion, while the Cl atoms gain an electron to become Cl− ions. This reaction involves the transfer of electrons between atoms.
The process of losing and gaining electrons occurs simultaneously. However, mentally we can separate the two processes. Oxidation is defined as the loss of one or more electrons by an atom. Reduction is defined as the gain of one or more electrons by an atom. O.I.L. R.I.G. is a useful mnemonic, standing for Oxidation Is Loss, Reduction Is Gain. So oxidation and reduction always occur together; it is only mentally that we can separate them. Chemical reactions that involve the transfer of electrons are called oxidation-reduction (or redox) reactions.
Redox reactions require that we keep track of the electrons assigned to each atom in a chemical reaction. How do we do that? We use oxidation numbers to keep track of electrons in atoms. Oxidation numbers are assigned to atoms based on four rules. Oxidation numbers are not necessarily equal to the charge on the atom (although sometimes they can be); we must keep the concepts of charge and oxidation numbers separate.
Assigning Oxidation Numbers
The rules for assigning oxidation numbers to atoms are not arbitrarily assigned, they are based on the relative electronegativity of the elements. The oxidation number tells us how many electrons the atom has gained or lost, relative to the neutral atom, as a result of chemically bonding to the other elements in the molecule.
Our rules for assigning oxidation numbers are as follows:
- Atoms in their elemental state are assigned an oxidation number of 0.
- If an atom is in it's elemental state it is either by itself or bonded to another atom of the same element, for example H2, O2, F2, or S8. In all of these cases the electronegativities of all of the atoms is the same and no one atom is going to be electronegative enough to steal an electron from another.
- Atoms in monatomic (i.e., one-atom) ions are assigned an oxidation number equal to their charge. Oxidation numbers are usually written with the sign first, then the magnitude, to differentiate them from charges.
- As the oxidation number tells us how many electrons the atom has gained or lost relative to the neutral atom and the charge on an ion tells us the same thing the charge is equal to the oxidation number for monatomic ions.
- Examples: Cu2+ has an oxidation number of +2, Cl- has an oxidation number of -1, O2- has an oxidation number of -2.
- In compounds, fluorine is assigned a −1 oxidation number; oxygen is usually assigned a −2 oxidation number [except in peroxide compounds (where it is −1) and in binary compounds with fluorine (where it is positive)]; and hydrogen is usually assigned a +1 oxidation number [except when it exists as the hydride ion (H−), in which case rule 2 prevails].
- Fluorine is the most electronegative element on the periodic table and wants one more electron to look like the nearest noble gas. With the highest electronegativity once fluorine is bonded to any other element its electronegativity allows it to take the extra electron it wants and fluorine will always have a -1 oxidation number if bonded to any other element.
- Oxygen is the second most electronegative element on the periodic table and wants two more electrons to look like the nearest noble gas. When bonding to anyone but fluorine (who is more electronegative) oxygen will take as many electrons as it can until it has 2 additional electrons and has an oxidation number of -2.
- In H2O oxygen will take one electron from each hydrogen, as each hydrogen only has one to offer, and have an oxidation number of -2, each hydrogen will have an oxidation number of +1.
- In H2O2 or H-O-O-H each hydrogen only has 1 electron available for the oxygens to take. Each oxygen gets a -1 oxidation number and each hydrogen gets a +1 oxidation number.
- In LiH, lithium is less electronegative than hydrogen. Hydrogen can either gain an electron to look like helium or it can give one up. As the hydrogen is less more electronegative than lithium the hydrogen has a -1 oxidation state and lithium has a +1 oxidation state in LiH.
- In compounds, all other atoms are assigned an oxidation number so that the sum of the oxidation numbers on all the atoms in the species equals the charge on the species (which is zero if the species is neutral).
- From drawing Lewis structures we know that the total number of electrons for any compound is the sum of the valence electrons plus or minus any charge on the molecule. The oxidation numbers tell us where in the molecule the electrons are living, but if we add up all the oxidation numbers they have to total to the charge on the molecule.
Here are some examples for practice. In H2, both H atoms have an oxidation number of 0 by rule 1. In MgCl2, magnesium has an oxidation number of +2, while chlorine has an oxidation number of −1 by rule 2. In H2O, the H atoms each have an oxidation number of +1, while the O atom has an oxidation number of −2, even though hydrogen and oxygen do not exist as ions in this compound (rule 3). By contrast, by rule 3, each H atom in hydrogen peroxide (H2O2) has an oxidation number of +1, while each O atom has an oxidation number of −1. We can use rule 4 to determine oxidation numbers for the atoms in SO2. Each O atom has an oxidation number of −2; for the sum of the oxidation numbers to equal the charge on the species (which is zero), the S atom is assigned an oxidation number of +4. Does this mean that the sulfur atom has a 4+ charge on it? No, it means only that the S atom is assigned a +4 oxidation number by our rules of apportioning electrons among the atoms in a compound.
Example \(\PageIndex{1}\)
Assign oxidation numbers to the atoms in each substance.
- Cl2
- GeO2
- Ca(NO3)2
Solution
- Cl2 is the elemental form of chlorine. Rule 1 states each atom has an oxidation number of 0.
- By rule 3, oxygen is normally assigned an oxidation number of −2. For the sum of the oxidation numbers to equal the charge on the species (zero), the Ge atom is assigned an oxidation number of +4.
-
Ca(NO3)2 can be separated into two parts: the Ca2+ ion and the NO3− ion. Considering these separately, the Ca2+ ion has an oxidation number of +2 by rule 2. Now consider the NO3− ion. Oxygen is assigned an oxidation number of −2, and there are three of them. According to rule 4, the sum of the oxidation numbers on all atoms must equal the charge on the species, so we have the simple algebraic equation
x + 3(−2) = −1where x is the oxidation number of the N atom and the −1 represents the charge on the species. Evaluating for x,
x + (−6) = −1x = +5Thus the oxidation number on the N atom in the NO3− ion is +5.
Exercise \(\PageIndex{1}\): Phosphoric Acid
Assign oxidation numbers to the atoms in H3PO4.
- Answer
-
H: +1; O: −2; P: +5
All redox reactions occur with a simultaneous change in the oxidation numbers of some atoms. At least two elements must change their oxidation numbers. When an oxidation number of an atom is increased in the course of a redox reaction, that atom is being oxidized. When an oxidation number of an atom is decreased in the course of a redox reaction, that atom is being reduced. Thus oxidation and reduction can also be defined in terms of increasing or decreasing oxidation numbers, respectively.
Example \(\PageIndex{2}\):
Identify what is being oxidized and reduced in this redox reaction.
\[\ce{2Na + Br2 → 2NaBr} \nonumber\]Solution
Both reactants are the elemental forms of their atoms, so the Na and Br atoms have oxidation numbers of 0. In the ionic product, the Na+ ions have an oxidation number of +1, while the Br− ions have an oxidation number of −1.
\[2\underset{0}{Na}+\underset{0}{Br_{2}}\rightarrow 2\underset{+1 -1}{NaBr} \nonumber\]
Sodium is increasing its oxidation number from 0 to +1, so it is being oxidized; bromine is decreasing its oxidation number from 0 to −1, so it is being reduced:
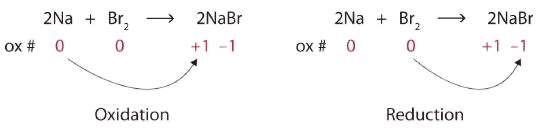
Because oxidation numbers are changing, this is a redox reaction. The total number of electrons being lost by sodium (two, one lost from each Na atom) is gained by bromine (two, one gained for each Br atom).
Exercise \(\PageIndex{2}\)
Identify what is being oxidized and reduced in this redox reaction.
\[\ce{C + O2 → CO2}\nonumber\]
- Answer
-
C is being oxidized from 0 to +4; O is being reduced from 0 to −2.
Oxidation reactions can become quite complex, as attested by the following redox reaction:
\[6H^{+}(aq)+2\underset{+7}{MnO_{4}^{-}}(aq)+5\underset{-1}{H_{2}O_{2}}(l)\rightarrow 2\underset{+2}{Mn^{2+}}(aq)+5\underset{0}{O_{2}}(g)+8H_{2}O(l)\]
To demonstrate that this is a redox reaction, the oxidation numbers of the species being oxidized and reduced are listed; can you determine what is being oxidized and what is being reduced? This is also an example of a net ionic reaction; spectator ions that do not change oxidation numbers are not displayed in the equation. Eventually, we will need to learn techniques for writing correct (i.e., balanced) redox reactions.
Food and Drink Application: Fortifying Food with Iron
Iron is an essential mineral in our diet; iron-containing compounds like the heme protein in hemoglobin could not function without it. Most biological iron has the form of the Fe2+ ion; iron with other oxidation numbers is almost inconsequential in human biology (although the body does contain an enzyme to reduce Fe3+ to Fe2+, so Fe3+ must have some biological significance, albeit minor). To ensure that we ingest enough iron, many foods are enriched with iron. Although Fe2+ compounds are the most logical substances to use, some foods—bread and breakfast cereals are the most well-known examples—use “reduced iron” as an ingredient. Reduced iron is simply iron metal; iron is added as a fine metallic powder. The metallic iron is oxidized to Fe2+ in the digestive system and then absorbed by the body, but the question remains: Why are we ingesting metallic iron? Why not just use Fe2+ salts as an additive?

Fig. 14.2.1 Ingredients Many prepared foods list reduced iron in their ingredients list.
Although it is difficult to establish conclusive reasons, a search of scientific and medical literature suggests a few reasons. One reason is that fine iron filings do not affect the taste of the product. The size of the iron powder (several dozen micrometers) is not noticeable when chewing iron-supplemented foods, and the tongue does not detect any changes in flavor that can be detected when using Fe2+ salts. Fe2+ compounds can affect other properties of foodstuffs during preparation and cooking, like dough pliability, yeast growth, and color. Finally, of the common iron substances that might be used, metallic iron is the least expensive. These factors appear to be among the reasons why metallic iron is the supplement of choice in some foods.
Key Takeaways
- Oxidation-reduction (redox) reactions involve the transfer of electrons from one atom to another.
- Oxidation numbers are used to keep track of electrons in atoms.
- There are rules for assigning oxidation numbers to atoms.
- Oxidation is an increase in oxidation number (loss of electrons); reduction is a decrease in oxidation number (gain of electrons).

