7.04: Single Displacement Reactions
- Page ID
- 178148
Learning Objectives
- Recognize chemical reactions as single-replacement reactions
- Use an activity series to predict whether single-replacement reactions will occur.
A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products. For example,
\[\ce{2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)}\]
is an example of a single-replacement reaction. The hydrogen atoms in \(\ce{HCl}\) are replaced by \(\ce{Zn}\) atoms, and in the process a new element-hydrogen-is formed. Another example of a single-replacement reaction is
\[\ce{2NaCl(aq) + F2(g) → 2NaF(s) + Cl2(g)}\]
Here the negatively charged ion changes from chloride to fluoride. A typical characteristic of a single-replacement reaction is that there is one element as a reactant and another element as a product.
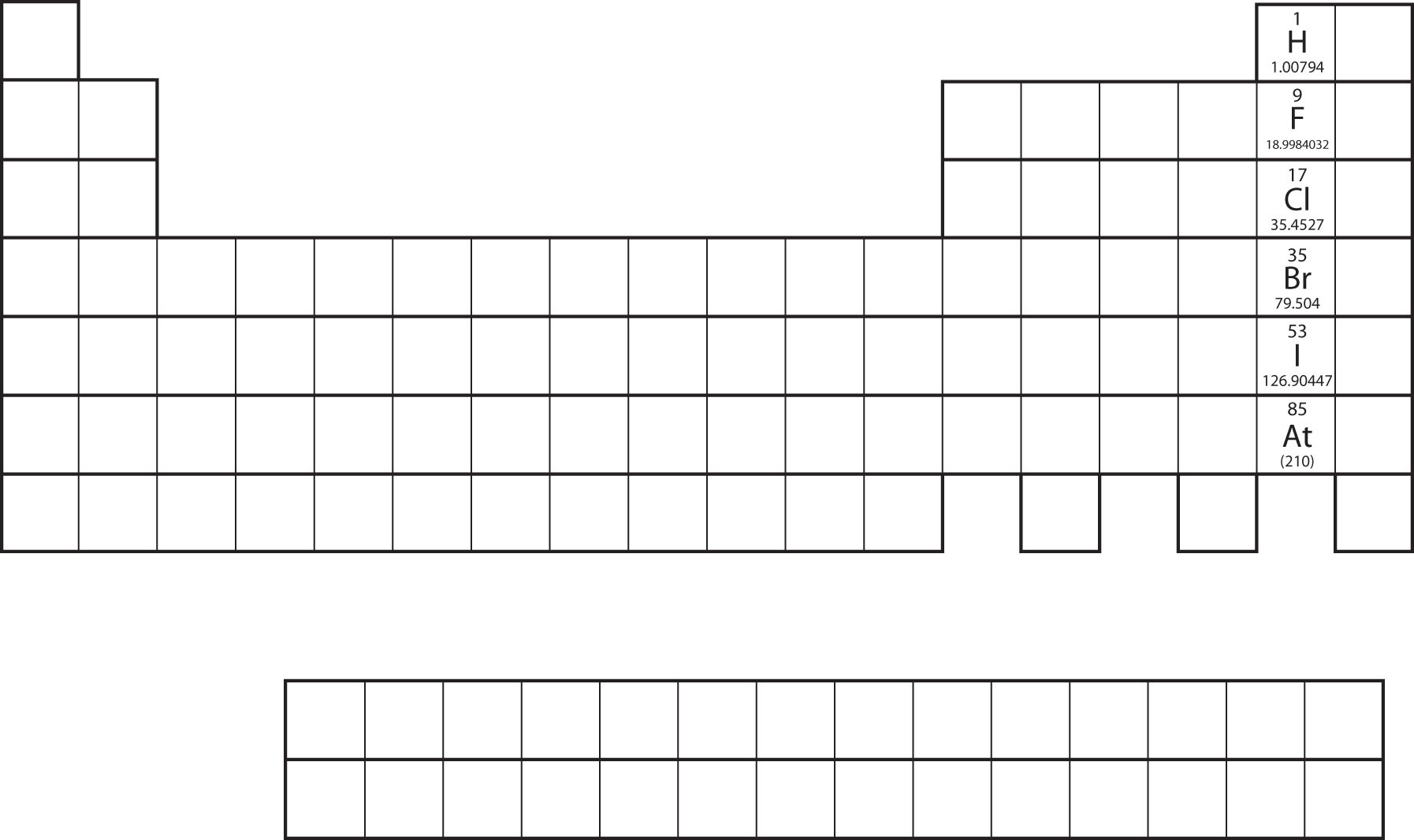
Not all proposed single-replacement reactions will occur between two given reactants. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. Collectively, these elements are called the halogens and are in the next-to-last column on the periodic table (Figure \(\PageIndex{1}\)). The elements on top of the column will replace the elements below them on the periodic table but not the other way around. Thus, the reaction represented by
\[\ce{CaI2(s) + Cl2(g) → CaCl2(s) + I2(s)}\]
will occur, but the reaction
\[\ce{CaF2(s) + Br2(ℓ) → CaBr2(s) + F2(g)}\]
will not because bromine is below fluorine on the periodic table. This is just one of many ways the periodic table helps us understand chemistry.
Example \(\PageIndex{1}\):
Will a single-replacement reaction occur? If so, identify the products.
- MgCl2 + I2 → ?
- CaBr2 + F2 → ?
Solution
- Because iodine is below chlorine on the periodic table, a single-replacement reaction will not occur.
- Because fluorine is above bromine on the periodic table, a single-replacement reaction will occur, and the products of the reaction will be CaF2 and Br2.
Exercise \(\PageIndex{1}\)
Will a single-replacement reaction occur? If so, identify the products.
\[\ce{FeI2 + Cl2 → }\]
Answer
Yes; FeCl2 and I2
Chemical reactivity trends are easy to predict when replacing anions in simple ionic compounds-simply use their relative positions on the periodic table. However, when replacing the cations, the trends are not as straightforward. This is partly because there are so many elements that can form cations; an element in one column on the periodic table may replace another element nearby, or it may not. A list called the activity series does the same thing the periodic table does for halogens: it lists the elements that will replace elements below them in single-replacement reactions. A simple activity series is shown below.
Activity Series for Cation Replacement in Single-Replacement Reactions
- Li
- K
- Ba
- Sr
- Ca
- Na
- Mg
- Al
- Mn
- Zn
- Cr
- Fe
- Ni
- Sn
- Pb
- H2
- Cu
- Hg
- Ag
- Pd
- Pt
- Au
Using the activity series is similar to using the positions of the halogens on the periodic table. An element on top will replace an element below it in compounds undergoing a single-replacement reaction. Elements will not replace elements above them in compounds.
Example \(\PageIndex{2}\)
Use the activity series to predict the products, if any, of each equation.
- FeCl2 + Zn → ?
- HNO3 + Au → ?
Solution
- Because zinc is above iron in the activity series, it will replace iron in the compound. The products of this single-replacement reaction are ZnCl2 and Fe.
- Gold is below hydrogen in the activity series. As such, it will not replace hydrogen in a compound with the nitrate ion. No reaction is predicted.
Exercise \(\PageIndex{2}\)
Use the activity series to predict the products, if any, of this equation.
\[\ce{AlPO4 + Mg → }\]
Answer
Mg3(PO4)2 and Al
Key Takeaways
- A single-replacement reaction replaces one element for another in a compound.
- The periodic table or an activity series can help predict whether single-replacement reactions occur.

