4.08 Ions
- Page ID
- 178134
Learning Objectives
- Know how ions form.
- Learn the characteristic charges that ions have.
So far, we have discussed elements and compounds that are electrically neutral. They have the same number of electrons as protons, so the negative charges of the electrons is balanced by the positive charges of the protons. However, this is not always the case. Electrons can move from one atom to another; when they do, species with overall electric charges are formed. Such species are called ions. Species with overall positive charges are termed cations, while species with overall negative charges are called anions. Remember that ions are formed only when electrons move from one atom to another; a proton never moves from one atom to another. Compounds formed from positive and negative ions are ionic compounds.
Individual atoms can gain or lose electrons. When they do, they become monatomic ions. When atoms gain or lose electrons, they usually gain or lose a characteristic number of electrons and so take on a characteristic overall charge. Figure \(\PageIndex{1}\) shows some common ions in terms of how many electrons they lose (making cations) or gain (making anions), as well as their positions on the periodic table. There are several things to notice about the ions in Figure \(\PageIndex{1}\). First, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. This is actually one of the chemical properties of metals and nonmetals: metals tend to form cations, while nonmetals tend to form anions. Second, elements that live in the first two columns and the last three columns of the period table show a defininte trend in charges. Every element in the first column forms a cation with charge 1+. Every element in the second column forms a cation with charge 2+. Elements in the third to last column almost all form an anion with a 2- charge and elements living in the second to last column almost all form anions with a 1- charge. The elements at the end of the periodic table do not form ions. We'll learn more about why this is the case in future chapters but for the time being if you can learn this trend it's fairly easy to determine the charge on most of the elements we see. Finally, most atoms form ions of a single characteristic charge. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the information in Figure \(\PageIndex{1}\) to memory, you will always know what charges most atoms form.
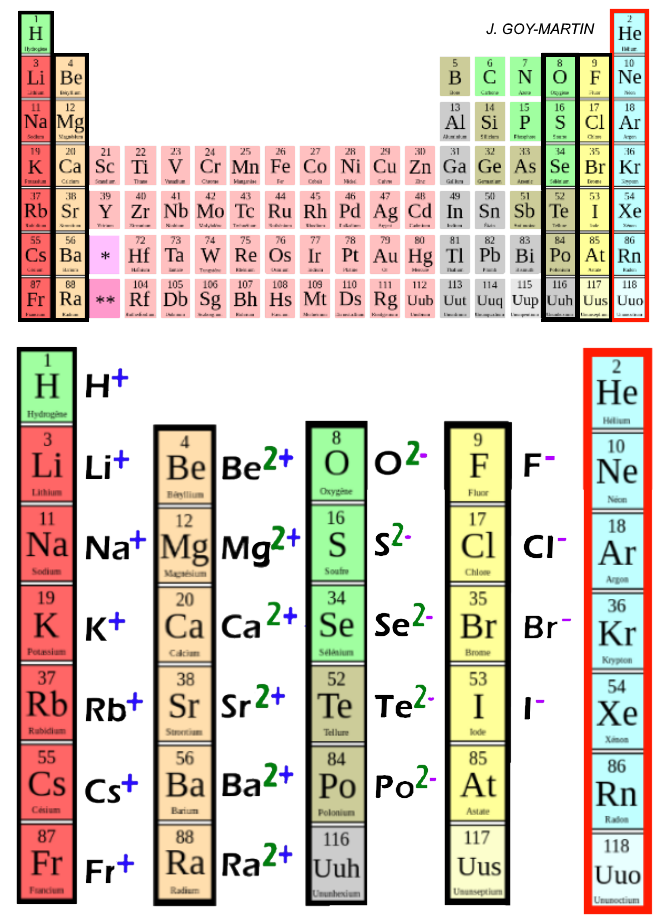
Figure \(\PageIndex{1}\): Groups on the periodic table and the charges on their ions, By Homme en Noir - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/inde...curid=66743988
While Figure \(\PageIndex{1}\) is helpful in determining the charge on a large number of our cations and anions it's hardly complete. A more complete table of ions and their charges can be found at Monotomic Ions of Various Charges. Examination of the table in the link given shows that there are some exceptions to the previous point. A few elements, all metals, can form more than one possible charge. For example, iron (Fe) atoms can form 2+ cations or 3+ cations. Cobalt (Co) is another element that can form more than one possible charged ion (2+ and 3+), while lead (Pb) can form 2+ or 4+ cations. Unfortunately, there is little understanding which two charges a metal atom may take, so it is best to just memorize the possible charges a particular element can have.
Note the convention for indicating an ion. The magnitude of the charge is listed as a right superscript next to the symbol of the element. If the charge is a single positive or negative one, the number 1 is not written; if the magnitude of the charge is greater than 1, then the number is written before the + or − sign. An element symbol without a charge written next to it is assumed to be the uncharged atom.
Naming ions
Naming an ion is straightforward. For a cation, simply use the name of the element and add the word ion (or if you want to be more specific, add cation) after the element’s name. So Na+ is the sodium ion; Ca2+ is the calcium ion. If the element has more than one possible charge, the value of the charge comes after the element name and before the word ion. Thus, Fe2+ is the iron two ion, while Fe3+ is the iron three ion. In print, we use roman numerals in parentheses to represent the charge on the ion, so these two iron ions would be represented as the iron(II) cation and the iron(III) cation, respectively.
For a monatomic anion, use the stem of the element name and append the suffix -ide to it, and then add ion. This is similar to how we named molecular compounds. Thus, Cl− is the chloride ion, and N3− is the nitride ion.
Example \(\PageIndex{1}\):
Name each species.
- O2−
- Co
- Co2+
Solution
- This species has a 2− charge on it, so it is an anion. Anions are named using the stem of the element name with the suffix -ide added. This is the oxide anion.
- Because this species has no charge, it is an atom in its elemental form. This is cobalt.
- In this case, there is a 2+ charge on the atom, so it is a cation. We note from Table 3.4.1 that cobalt cations can have two possible charges, so the name of the ion must specify which charge the ion has. This is the cobalt(II) cation.
Exercise \(\PageIndex{1}\)
Name each species.
- P3−
- Sr2+
Answers
- the phosphide anion
- the strontium cation
Key Takeaways
- Ions form when atoms lose or gain electrons.

