Acetals as Protecting Groups
The importance of acetals as carbonyl derivatives lies chiefly in their stability and lack of reactivity in neutral to strongly basic environments. As long as they are not treated by acids, especially aqueous acid, acetals exhibit all the lack of reactivity associated with ethers in general. Among the most useful and characteristic reactions of aldehydes and ketones is their reactivity toward strongly nucleophilic (and basic) metallo-hydride, alkyl and aryl reagents. If the carbonyl functional group is converted to an acetal these powerful reagents have no effect; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented.
In the following example we would like a Grignard reagent to react with the ester and not the ketone. This cannot be done without a protecting group because Grignard reagents react with esters and ketones.
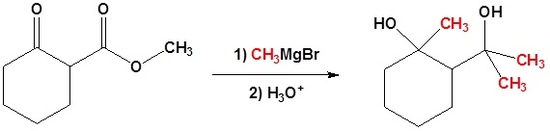
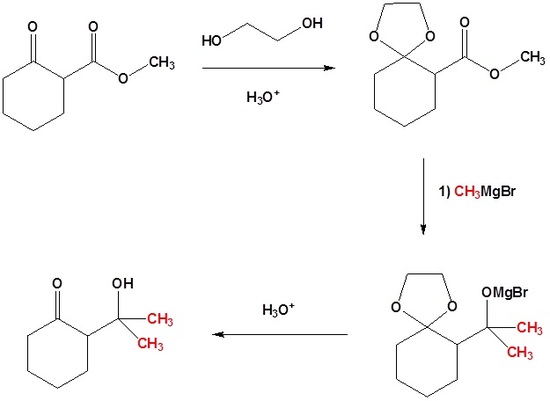
Compound 1.4.1.4A illustrates several important points in Protection / Deprotection protocol. Both the functional groups could react with a Grignard Reagent. Carboxylic acid group would first react with one mole of the Grignard Reagent to give a carboxylate anion salt. This anion does not react any further with the reagent. When two moles of Grignard Reagent are added to the reaction mixture, the second mole attacks the ketone to give a tertiary alcohol. On aqueous work-up, the acid group is regenerated. Thus, the first mole of the reagent provides a selective transient protection for the –COOH group. Once the acid group is esterified, such selectivity towards this reagent is lost. The reagent attacks at both sites. If reaction is desired only at the ester site, the keto- group should be selectively protected as an acetal. In the next step, the grignard reaction is carried out. Now the reagent has only one group available for reaction. On treatment with acid, the ketal protection in the intermediate compound is also hydrolyzed to regenerated the keto- group.
Fig 1.4.1.4
Protection of Aldehydes and Ketones
Since alcohols, aldehydes and ketones are the most frequently manipulated functional groups in organic synthesis, a great deal of work has appeared in their protection / deprotection strategies. In this discussion let us focus on the classes of protecting groups rather than an exhaustive treatment of all the protections.
Acetals
There are two general methods for the introduction of this protection. Transketalation is the method of choice when acetals (ketals) with methanol are desired. Acetone is the by-product, which has to be removed to shift the equilibrium to the right hand side. This is achieved by refluxing with a large excess of the acetonide reagent. Acetone formed is constantly distilled. In the case of cyclic diols, the water formed is continuously removed using a Dean-Stork condenser (Fig 1.4.1.6).

Fig 1.4.1.6
The rate of formation of ketals from ketones and 1,2-ethanediol (ethylene glycol), 1,3-propanediol and 2,2-dimethyl-1,3-propanediol are different. So is the deketalation reaction. This has enabled chemists to selectively work at one center. The following examples from steroid chemistry illustrate these points (Fig 1.4.1.7).

Fig 1.4.1.7
The demand for Green Chemistry processes has prompted search for new green procedures. Some examples from recent literature are given here (Fig 1.4.1.8).

Fig 1.4.1.8
Thioketals
Compared with their oxygen analogues, thioketals markedly differ in their chemistry. The formation as well as deprotection is promoted by suitable Lewis acids. The thioacetals are markedly stable under deketalation conditions, thus paving way for selective operations at two different centers. When conjugated ketones are involved, the ketal formation (as well as deprotection) proceeds with double bond migration. On the other hand, thioketals are formed and deketalated without double bond migration (Fig 1.4.1.9).
Fig 1.4.1.9
Silyl Ethers (R – OSiR3)
The oxygen – silicon sigma bond is stable to lithium and Grignard reagents, nucleophiles and hydride reagents but very unstable to water and mild aqueous acid and base conditions. A silyl ether of secondary alcohol is less reactive than that of a primary alcohol. The O – trimethylsilyl (O – SiMe3) was first protection of this class. (Fig 1.4.1.24).
Fig 1.4.1.24
Replacement of methyl group with other alkyl and aryl groups gives a large variety of silyl ether with varying degrees of stability towards hydrolysis (Fig 1.4.1.25).
Fig 1.4.1.25
The following examples illustrate the selectivity in formation and hydrolysis of this group (Fig 1.4.1.26).
Fig 1.4.1.26
Contributors
- Prof. R Balaji Rao (Department of Chemistry, Banaras Hindu University, Varanasi) as part of Information and Communication Technology











