Chapter 6.1: Naming Binary Covalent Compounds
- Page ID
- 18848
Learning Objectives
- To name covalent compounds that contain up to three elements.
As with ionic compounds, the system that chemists have devised for naming covalent compounds enables us to write the molecular formula from the name and vice versa. In this and the following section, we describe the rules for naming simple covalent compounds. We begin with inorganic compounds and then turn to simple organic compounds that contain only carbon and hydrogen.
Binary Inorganic Compounds
Binary covalent compounds—that is, covalent compounds that contain only two elements—are named using a procedure similar to that used to name simple ionic compounds, but prefixes are added as needed to indicate the number of atoms of each kind. The procedure, diagrammed in Figure 6.1.1, uses the following steps:
Figure 6.1.1 Naming a Covalent Inorganic Compound
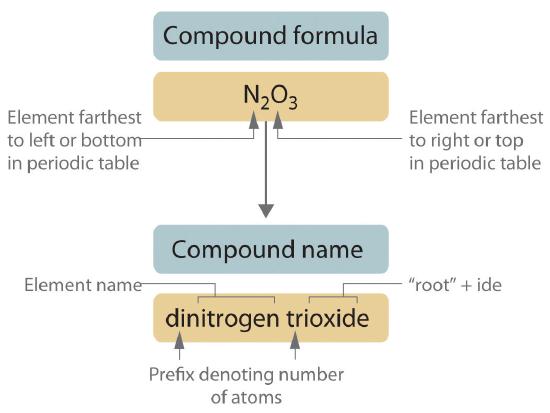
-
Place the elements in their proper order.
- The element farthest to the left in the periodic table is usually named first. If both elements are in the same group, the element closer to the bottom of the column is named first.
- The second element is named as if it were a monatomic anion in an ionic compound (even though it is not), with the suffix -ide attached to the root of the element name.
The reason for this is that we know that the less electronegative elements are to the left and bottom of the periodic table. The more electronegative elements attract electrons from the less electronegative ones and behave more like anions than the less electronegative ones that behave like cations. However, unlike binary inorganic compounds electron transfer is not complete and the binary organic compounds do not form crystal lattices,
2. Identify the number of each type of atom present.
-
-
Prefixes derived from Greek stems are used to indicate the number of each type of atom in the formula unit (Table 6.1.1). The prefix mono- (“one”) is used only when absolutely necessary to avoid confusion, just as we omit the subscript 1 when writing molecular formulas.
To demonstrate steps 1 and 2a, we name HCl as hydrogen chloride (because hydrogen is to the left of chlorine in the periodic table) and PCl5 as phosphorus pentachloride. The order of the elements in the name of BrF3, bromine trifluoride, is determined by the fact that bromine lies below fluorine in group 17.
Table 6.1.1 Prefixes for Indicating the Number of Atoms in Chemical Names
Prefix Number mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa- 6 hepta- 7 octa- 8 nona- 9 deca- 10 undeca- 11 dodeca- 12 - If a molecule contains more than one atom of both elements, then prefixes are used for both. Thus N2O3 is dinitrogen trioxide, as shown in Figure 6.1.1.
- In some names, the final a or o of the prefix is dropped to avoid awkward pronunciation. Thus OsO4 is osmium tetroxide rather than osmium tetraoxide.
-
-
Write the name of the compound.
- Binary compounds of the elements with oxygen are generally named as “element oxide,” with prefixes that indicate the number of atoms of each element per formula unit. For example, CO is carbon monoxide. The only exception is binary compounds of oxygen with fluorine, which are named as oxygen fluorides following the rules for the most electronegative element being treated as the anion for naming purposes.
- Certain compounds are always called by the common names that were assigned long ago when names rather than formulas were used. For example, H2O is water (not dihydrogen oxide); NH3 is ammonia; PH3 is phosphine; SiH4 is silane; and B2H6, a dimer of BH3, is diborane. For many compounds, the systematic name and the common name are both used frequently, so you must be familiar with them. For example, the systematic name for NO is nitrogen monoxide, but it is much more commonly called nitric oxide. Similarly, N2O is usually called nitrous oxide rather than dinitrogen monoxide. Notice that the suffixes -ic and -ous are the same ones used for ionic compounds.
Note the Pattern
Start with the element at the far left in the periodic table and work to the right. If two or more elements are in the same group, start with the bottom element and work up.
Example 1
Write the name of each binary covalent compound.
- SF6
- N2O4
- ClO2
Given: molecular formula
Asked for: name of compound
Strategy:
A List the elements in order according to their positions in the periodic table. Identify the number of each type of atom in the chemical formula and then use Table 6.1.1 to determine the prefixes needed.
B If the compound contains oxygen, follow step 3a. If not, decide whether to use the common name or the systematic name.
Solution:
- A Because sulfur is to the left of fluorine in the periodic table, sulfur is named first. Because there is only one sulfur atom in the formula, no prefix is needed. B There are, however, six fluorine atoms, so we use the prefix for six: hexa- (Table 6.4.1). The compound is sulfur hexafluoride.
- A Because nitrogen is to the left of oxygen in the periodic table, nitrogen is named first. Because more than one atom of each element is present, prefixes are needed to indicate the number of atoms of each. According to Table 6.4.1, the prefix for two is di-, and the prefix for four is tetra-. B The compound is dinitrogen tetroxide (omitting the a in tetra- according to step 2c) and is used as a component of some rocket fuels.
- A Although oxygen lies to the left of chlorine in the periodic table, it is not named first because ClO2 is an oxide of an element other than fluorine (step 3a). Consequently, chlorine is named first, but a prefix is not necessary because each molecule has only one atom of chlorine. B Because there are two oxygen atoms, the compound is a dioxide. Thus the compound is chlorine dioxide. It is widely used as a substitute for chlorine in municipal water treatment plants because, unlike chlorine, it does not react with organic compounds in water to produce potentially toxic chlorinated compounds.
Exercise
Write the name of each binary covalent compound.
- IF7
- N2O5
- OF2
Answer:
- iodine heptafluoride
- dinitrogen pentoxide
- oxygen difluoride
Example 2
Write the formula for each binary covalent compound.
- sulfur trioxide
- diiodine pentoxide
Given: name of compound
Asked for: formula
Strategy:
List the elements in the same order as in the formula, use Table 6.4.1 to identify the number of each type of atom present, and then indicate this quantity as a subscript to the right of that element when writing the formula.
Solution:
- Sulfur has no prefix, which means that each molecule has only one sulfur atom. The prefix tri- indicates that there are three oxygen atoms. The formula is therefore SO3. Sulfur trioxide is produced industrially in huge amounts as an intermediate in the synthesis of sulfuric acid.
- The prefix di- tells you that each molecule has two iodine atoms, and the prefix penta- indicates that there are five oxygen atoms. The formula is thus I2O5, a compound used to remove carbon monoxide from air in respirators.
Exercise
Write the formula for each binary covalent compound.
- silicon tetrachloride
- disulfur decafluoride
Answer:
- SiCl4
- S2F10
The structures of some of the compounds in Example 8 and Example 9 are shown in Figure 6.4.2 ", along with the location of the “central atom” of each compound in the periodic table. The compositions and structures of such compounds can be deduced from the rules for covalent bonding, Lewis structures, hybridization, expanded octets and VSEPR discussed in Chapters 4, 5 and 6. and but this is not true.
Figure 6.1.2 The Structures of Some Covalent Inorganic Compounds and the Locations of the “Central Atoms” in the Periodic Table
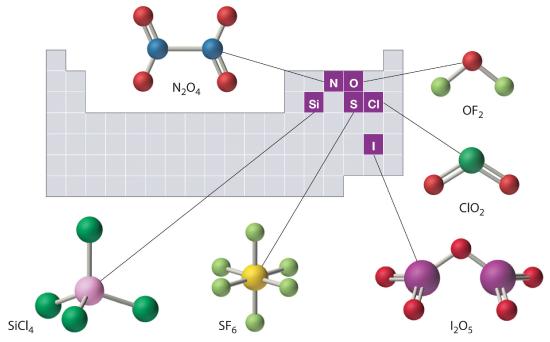
The compositions and structures of covalent inorganic compounds are not random. As you learned in Chapter 4 and Chapter 5, they can be predicted from the locations of the component atoms in the periodic table.
Summary
Covalent inorganic compounds are named by a procedure similar to that used for ionic compounds, using prefixes to indicate the numbers of atoms in the molecular formula. The simplest organic compounds are the hydrocarbons, which contain only carbon and hydrogen. Alkanes contain only carbon–hydrogen and carbon–carbon single bonds, alkenes contain at least one carbon–carbon double bond, and alkynes contain one or more carbon–carbon triple bonds. Hydrocarbons can also be cyclic, with the ends of the chain connected to form a ring. Collectively, alkanes, alkenes, and alkynes are called aliphatic hydrocarbons. Aromatic hydrocarbons, or arenes, are another important class of hydrocarbons that contain rings of carbon atoms related to the structure of benzene (C6H6). A derivative of an alkane or an arene from which one hydrogen atom has been removed is called an alkyl group or an aryl group, respectively. Alcohols are another common class of organic compound, which contain an –OH group covalently bonded to either an alkyl group or an aryl group (often abbreviated R).
Key Takeaway
- Covalent inorganic compounds are named using a procedure similar to that used for ionic compounds.
Conceptual Problems
-
Name each compound.
- NiO
- TiO2
- N2O
- CS2
- SO3
- NF3
- SF6
-
Name each compound.
- HgCl2
- IF5
- N2O5
- Cl2O
- HgS
- PCl5
-
-
-
For each structural formula, write the condensed formula and the name of the compound.
-
-
Would you expect PCl3 to be an ionic compound or a covalent compound? Explain your reasoning.
-
Answer
Numerical Problems
-
Write the formula for each compound.
- dinitrogen monoxide
- silicon tetrafluoride
- boron trichloride
- nitrogen trifluoride
- phosphorus tribromide
-
Write the formula for each compound.
- dinitrogen trioxide
- iodine pentafluoride
- boron tribromide
- oxygen difluoride
- arsenic trichloride
-
Write the formula for each compound.
- thallium(I) selenide
- neptunium(IV) oxide
- iron(II) sulfide
- copper(I) cyanide
- nitrogen trichloride
-
Name each compound.
- RuO4
- PbO2
- MoF6
- Hg2(NO3)2·2H2O
- WCl4
-
Name each compound.
- NbO2
- MoS2
- P4S10
- Cu2O
- ReF5
Answers
-
- N2O
- SiF4
- BCl3
- NF3
- PBr3
-
- Tl2Se
- NpO2
- FeS
- CuCN
- NCl3
-
- niobium (IV) oxide
- molybdenum (IV) sulfide
- tetraphosphorus decasulfide
- copper(I) oxide
- rhenium(V) fluoride
Contributors
- Anonymous
Modified by Joshua Halpern