13.3: Chemical and Biological Contamination
- Last updated
- Save as PDF
- Page ID
- 303983
Learning Objective
- Describe the impact of human activities on water quality.
The global water crisis also involves water pollution. For water to be useful for drinking and irrigation, it must not be polluted beyond certain thresholds. According to the World Health Organization, in 2008 approximately 880 million people in the world (or 13% of world population) did not have access to safe drinking water. At the same time, about 2.6 billion people (or 40% of world population) lived without improved sanitation, which is defined as having access to a public sewage system, septic tank, or even a simple pit latrine. Each year approximately 1.7 million people die from diarrheal diseases associated with unsafe drinking water, inadequate sanitation, and poor hygiene. Almost all of these deaths are in developing countries, and around 90% of them occur among children under the age of 5 (Figure 1). Compounding the water crisis is the issue of social justice; poor people more commonly lack clean water and sanitation than wealthy people in similar areas. Globally, improving water safety, sanitation, and hygiene could prevent up to 9% of all disease and 6% of all deaths.
In addition to the global waterborne disease crisis, chemical pollution from agriculture, industry, cities, and mining threatens global water quality. In Gallup public polls conducted over the past decade Americans consistently put water pollution and water supply as the top environmental concerns over issues such as air pollution, deforestation, species extinction, and global warming.
Water pollution is the contamination of water by an excess amount of a substance that can cause harm to human beings and/or the ecosystem. The level of water pollution depends on the abundance of the pollutant, the ecological impact of the pollutant, and the use of the water. Pollutants are derived from biological, chemical, or physical processes. Although natural processes such as volcanic eruptions or evaporation sometimes can cause water pollution, most pollution is derived from human, land-based activities. Water pollutants can move through different water reservoirs, as the water carrying them progresses through stages of the water cycle.
Pollutants enter water supplies from point sources, which are readily identifiable and relatively small locations, or nonpoint sources, which are large and more diffuse areas (Figure \(\PageIndex{1}\)) . Point sources of pollution include animal factory farms that raise a large number and high density of livestock such as cows, pigs, and chickens. Also, pipes included are pipes from a factories or sewage treatment plants. Combined sewer systems that have a single set of underground pipes to collect both sewage and storm water runoff from streets for wastewater treatment can be major point sources of pollutants. During heavy rain, storm water runoff may exceed sewer capacity, causing it to back up and spilling untreated sewage directly into surface waters.

.jpg?revision=1&size=bestfit&width=503&height=338)
This section and the next will focus on how human activities affect water quality.
Too Much Organic Matter Means Too Little Oxygen
Oxygen-demanding waste is an extremely important pollutant to ecosystems. Most surface water in contact with the atmosphere has a small amount of dissolved oxygen, which is needed by aquatic organisms for cellular respiration. Bacteria decompose dead organic matter and remove dissolved oxygen (O2) according to the following reaction:
\[\text{organic matter} + O_{2} \rightarrow CO_{2} + H_{2} O \nonumber \]
Too much decaying organic matter in water is a pollutant because it removes oxygen from water, which can kill fish, shellfish, and aquatic insects. The amount of oxygen used by aerobic (in the presence of oxygen) bacterial decomposition of organic matter is called biochemical oxygen demand (BOD). The major source of dead organic matter in many natural waters is sewage; grass and leaves are smaller sources. An unpolluted water body with respect to BOD is a turbulent river that flows through a natural forest. Turbulence continually brings water in contact with the atmosphere where the O2 content is restored. The dissolved oxygen content in such a river ranges from 10 to 14 ppm O2, BOD is low, and clean-water fish such as trout. A polluted water body with respect to oxygen is a stagnant deep lake in an urban setting with a combined sewer system. This system favors a high input of dead organic carbon from sewage overflows and limited chance for water circulation and contact with the atmosphere. In such a lake, the dissolved O2 content is ≤5 ppm O2, BOD is high, and low O2-tolerant fish, such as carp and catfish dominate.
Excessive plant nutrients, particularly nitrogen (N) and phosphorous (P), are pollutants closely related to oxygen-demanding waste. Aquatic plants require about 15 nutrients for growth, most of which are plentiful in water. N and P are called limiting nutrients, however, because they usually are present in water at low concentrations and therefore restrict the total amount of plant growth. This explains why N and P are major ingredients in most fertilizer. High concentrations of N and P from human sources (mostly agricultural and urban runoff including fertilizer, sewage, and phosphorus-based detergent) can cause cultural eutrophication, which leads to the rapid growth of aquatic producers, particularly algae. Thick mats of floating algae or rooted plants lead to a form of water pollution that damages the ecosystem by clogging fish gills and blocking sunlight. A small percentage of algal species produce toxins that can kill animals, including humans. Exponential growths of these algae are called harmful algal blooms. When the prolific algal layer dies, it becomes oxygen-demanding waste, which can create very low O2 concentrations in the water (< 2 ppm O2), a condition called hypoxia. This results in a dead zone because it causes death from asphyxiation to organisms that are unable to leave that environment. An estimated 50% of lakes in North America, Europe, and Asia are negatively impacted by cultural eutrophication. In addition, the size and number of marine hypoxic zones have grown dramatically over the past 50 years including a very large dead zone located offshore Louisiana in the Gulf of Mexico. Cultural eutrophication and hypoxia are difficult to combat, because they are caused primarily by nonpoint source pollution, which is difficult to regulate, and N and P, which are difficult to remove from wastewater.
Eutrophication is an increase in the concentration of chemical nutrients in an ecosystem to an extent that increases the primary productivity of the ecosystem. Depending on the degree of eutrophication, subsequent negative environmental effects such as anoxia (oxygen depletion) and severe reductions in water quality may occur, affecting fish and other animal populations.
Chemical Pollution from Waste
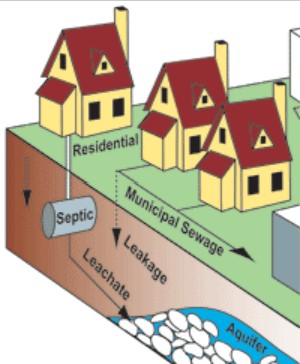
Since the 1990s, water contamination by pharmaceuticals has been an environmental issue of concern. In addition, it is important to note that many public health professionals in the United States began writing reports of pharmaceutical contamination in waterways in the 1970s.” Most pharmaceuticals are deposited in the environment through human consumption and excretion, and are often filtered ineffectively by municipal sewage treatment plants which are not designed to manage them (Figure \(\PageIndex{2}\)) . Once in the water, they can have diverse, subtle effects on organisms, although research is still limited. Pharmaceuticals may also be deposited in the environment through improper disposal, runoff from sludge fertilizer and reclaimed wastewater irrigation, and leaky sewer pipes. In 2009, an investigative report by Associated Press concluded that U.S. manufacturers had legally released 271 million pounds of compounds used as drugs into the environment, 92% of which was the industrial chemicals phenol and hydrogen peroxide, which are also used as antiseptics. It could not distinguish between drugs released by manufacturers as opposed to the pharmaceutical industry. It also found that an estimated 250 million pounds of pharmaceuticals and contaminated packaging were discarded by hospitals and long-term care facilities.

The use of pharmaceuticals and personal care products (PPCPs) is on the rise with an estimated increase from 2 billion to 3.9 billion annual prescriptions between 1999 and  2009 in the United States alone. Figure \(\PageIndex{3}\) illustrates how PPCPs enter into the environment through individual human activity and as residues from manufacturing, agribusiness, veterinary use, and hospital and community use. In Europe, the input of pharmaceutical residues via domestic waste water is estimated to be around 80% whereas 20% is coming from hospitals. Individuals may add PPCPs to the environment through waste excretion and bathing as well as by directly disposing of unused medications to septic tanks, sewers, or trash. Because PPCPs tend to dissolve relatively easily and do not evaporate at normal temperatures, they often end up in soil and water bodies.
2009 in the United States alone. Figure \(\PageIndex{3}\) illustrates how PPCPs enter into the environment through individual human activity and as residues from manufacturing, agribusiness, veterinary use, and hospital and community use. In Europe, the input of pharmaceutical residues via domestic waste water is estimated to be around 80% whereas 20% is coming from hospitals. Individuals may add PPCPs to the environment through waste excretion and bathing as well as by directly disposing of unused medications to septic tanks, sewers, or trash. Because PPCPs tend to dissolve relatively easily and do not evaporate at normal temperatures, they often end up in soil and water bodies.
Some PPCPs are broken down or processed easily by a human or animal body and/or degrade quickly in the environment . However, others do not break down or degrade easily. The likelihood or ease with which an individual substance will break down depends on its chemical makeup and the metabolic pathway of the compound.
While the full effects of most PPCPs on the environment are not understood, there is concern about the potential they have for harm because they may act unpredictably when mixed with other chemicals from the environment or concentrate in the food chain. Additionally, some PPCPs are active at very low concentrations, and are often released continuously in large or widespread quantities.
Because of the high solubility of most PPCPs, aquatic organisms are especially vulnerable to their effects. The increased presence of estrogen and other synthetic hormones in waste water due to birth control and hormonal therapies has been linked to increased feminization of exposed fish and other aquatic organisms. The chemicals within these PPCP products could either affect the feminization or masculinization of different fishes, therefore affecting their reproductive rates.
The major route for pharmaceutical residues to reach the aquatic environment is most probably by excretion from patients undergoing pharma treatment. Since many pharmaceutical substances are not metabolized in the body they may be excreted in biologically active form, usually via the urine. Furthermore, many pharmaceutical substances are not fully taken up from the intestine (following oral administration in patients) into their blood stream. The fraction not taken up into the blood stream will remain in the gut and eventually be excreted via the faeces. Hence, both urine and faeces from treated patients contain pharmaceutical residues. Between 30 and 90% of the orally administered dose is generally excreted as active substance in the urine.
An additional source to environmental pollution with pharmaceuticals is improper disposal of unused or expired drug residues. In European countries take-back systems for such residues are usually in place (although not always utilized to full extent) while in e.g. the US only voluntary initiatives on a local basis exist. Though most of the waste goes to incineration and people are asked to throw unused or expired pharmaceuticals into the household waste investigations in Germany showed that up to 24% of liquid pharmaceuticals and 7% of tablets or ointments are disposed always or at least “rarely” via the toilet or sink.
Proper destruction of pharma residues should yield rest products without any pharmaceutical or ecotoxic activity. Furthermore, the residues should not act as components in the environmental formation of new such products. Incineration at a high temperature (>1000 degrees Celsius) is considered to fulfill the requirements, but even following such incineration residual ashes from the incineration should be properly taken care of.
Pharmaceuticals used in veterinary medicine, or as additives to animal food, pose a different problem, since they are excreted into soil or possibly open surface waters. It is well known that such excretions may affect terrestrial organisms directly, leading to extinction of exposed species (e.g. dung-beetles). Lipid-soluble pharma residues from veterinary use may bind strongly to soil particles, with little tendency to leak out to ground water or to local surface waters. More water-soluble residues may be washed out with rain or melting snow and reach both ground water and surface water streams.
Water Borne Diseases
Waterborne diseases are conditions caused by pathogenic micro-organisms that are transmitted in water. These diseases can be spread while bathing, washing, drinking water, or by eating food exposed to contaminated water. While diarrhea and vomiting are the most commonly reported symptoms of waterborne illness, other symptoms can include skin, ear, respiratory, or eye problems. Waterborne diseases are impacted by a country's economy and also impact the economy by being costly to deal with.
Microorganisms causing diseases that characteristically are waterborne prominently include protozoa and bacteria, many of which are intestinal parasites, or invade the tissues or circulatory system through walls of the digestive tract. Various other waterborne diseases are caused by viruses. (In spite of philosophical difficulties associated with defining viruses as "organisms", it is practical and convenient to regard them as microorganisms in this connection.)
Yet other important classes of water-borne diseases are caused by metazoan parasites. Typical examples include certain Nematoda, that is to say "roundworms". As an example of water-borne Nematode infections, one important waterborne nematode disease is Dracunculiasis. It is acquired by swallowing water in which certain copepoda occur that act as vectors for the Nematoda. Anyone swallowing a copepod that happens to be infected with Nematode larvae in the genus Dracunculus, becomes liable to infection. The larvae cause guinea worm disease.
Another class of waterborne metazoan pathogens are certain members of the Schistosomatidae, a family of blood flukes. They usually infect victims that make skin contact with the water. Blood flukes are pathogens that cause Schistosomiasis of various forms, more or less seriously affecting hundreds of millions of people worldwide.
Video \(\PageIndex{1}\) The Coalition for Global Community Health is working within existing social structures in Belén, Iquitos, Peru to uphold the human rights of the community members. We speak directly with community members in an open forum to learn about their needs, desires, and ideas for creating an opportunity to change their communities for the better
The table below shows water-borne diseases that can result from viruses, bacteria, and parasites. In some cases, vaccines are available. When eating, drinking, or swimming, it is important to be aware of how you could be affected by these pathogens. Sanitation of drinking water with chlorine-based compounds reduces the power of these pathogens. In addition, proper handling of foods and beverages could reduce your risk of developing one or more of the following health problems.
| Pathogen Name | Pathogen Type | Source | Health problem | Prevention/Treatment |
|---|---|---|---|---|
| Giardia | Parasite | Fecal contamination and uncooked food | Vomiting, diarrhea, and cramps | Medication afterward |
| Cryptosporidium | Parasite | Fecal contamination | Vomiting, diarrhea, fever, and cramps | Medication afterward |
| Typhoid | Bacteria | Fecal contamination | High fever, stomach pains, headache, and rash | Vaccination/Antibiotics |
| E. coli | Bacteria | Fecal contamination | Diarrhea and cramps | Fluids |
| Legionella | Bacteria | Found naturally in heated water | Causes Legionnaires (a type of pneumonia) | Medications afterward |
| Cholera | Bacteria | Related to fecal contamination or undercooked or raw shellfish | Diarrhea | Vaccine/Rehydration, antibiotics, and Zinc |
| Hepatitis A | Virus | Contaminated food and water | Vomiting, dark urine, and yellowing of the eyes. | Vaccination/Fluids |
| Polio | Virus | Fecal contamination | Flu symptoms, paralysis | Vaccination |
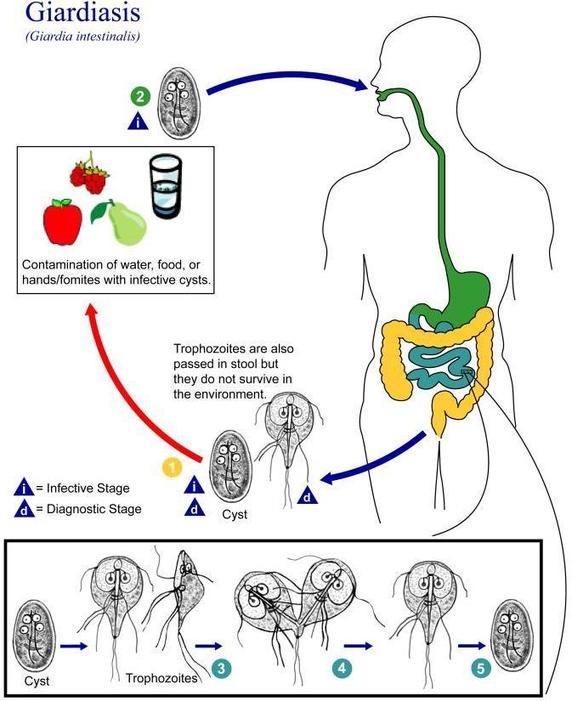
(Figure \(\PageIndex{4}\)) shows how a person might contract Giardiasis from giardia, a parasite. This particular pathogen can live in a body up to six months. Once detected through a stool sample, a patient can be prescribed specific antibiotics like Flagyl to treat the infection. Unfortunately, there is no vaccine for preventing Giardiasis.
Acidic Waters
Water can become contaminated at any part of the water cycle. Air pollution can affect water vapor and water liquid. Combustion sources like vehicles and power plants generate compounds like \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\), and other various inorganic and organic volatile organic compounds (VOC) species (Table \(\PageIndex{2}\)). Some of these compounds can become soluble in water. This could affect pH (or acidity) level of water surface water. Normally, the pH of water is a neutral value (or pH= 7). When \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\) enter the water cycle, then the pH level is lowered below 7.0. If these gases are absorbed in rain clouds, then acid rain results. Specific acids involved in acid rain are sulfuric, nitric, and carbonic. This environmental problem affects living organisms and building materials. Acid solutions can corrode metals and make them soluble as well.
| Combustion products | Sources |
|---|---|
| CO2 and CO | Combustion of any material (any fuel or tree) |
| NOX (NO2 and NO3) | High-temperature combustion of any fuel ( gas, diesel, or coal), a product of lightning |
| SOx (SO2 and SO3) | Combustion of sulfur-based fuels (diesel and coal), volcanic release |
| VOC (volatile organic compound) | Combustion of any carbon-based fuel (gas, diesel, or coal), fumes from paints or solvents |
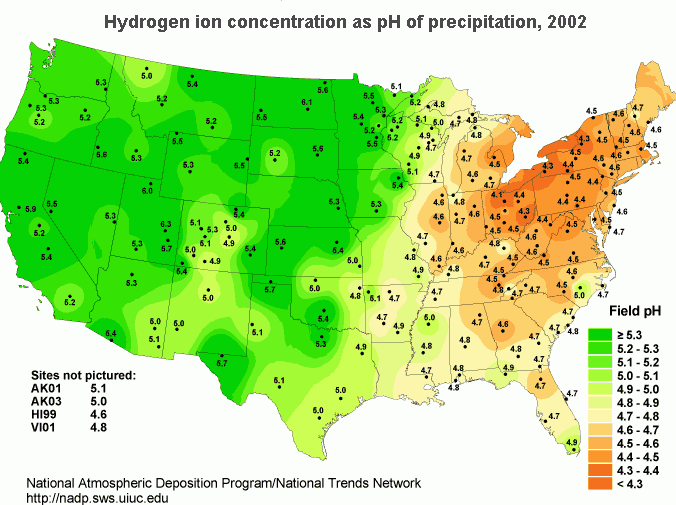
In the United States, the northeast has the most problems with acid rain. Concentrated populations that use electrical energy and vehicles contribute greatly to the pH reduction of rainwater. Reducing gaseous output requires capping combustion sources (vehicles and power plants). Acidity in rain is measured by collecting samples of rain and measuring its pH. To find the distribution of rain acidity, weather conditions are monitored and rain samples are collected at sites all over the country (Figure \(\PageIndex{5}\)). The areas of greatest acidity (lowest pH values) are located in the Northeastern United States. This pattern of high acidity is caused by a large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast. In addition, the prevailing wind direction brings storms and pollution to the Northeast from the Midwest, and dust from the soil and rocks in the Northeastern United States is less likely to neutralize acidity in the rain.
Summary
- Water pollution is the contamination of water by an excess amount of a substance that can cause harm to human beings and/or the ecosystem.
- Pollutants enter water supplies from point sources, which are readily identifiable and relatively small locations, or nonpoint sources, which are large and more diffuse areas
- Organic matter as well as phosphates and nitrates from human and farm animal waste support the growth of algae and microorganisms, including bacteria.
- Eutrophication is an increase in the concentration of chemical nutrients in an ecosystem to an extent that increases the primary productivity of the ecosystem. Depending on the degree of eutrophication, subsequent negative environmental effects such as anoxia (oxygen depletion) and severe reductions in water quality may occur, affecting fish and other animal populations.
- Acid rain (formed as a consequence of air pollution) could affect building materials and living organisms on land and various bodies of water.
Contributors and Attributions
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher.
- Wikipedia
- US EPA

