10.6: Organic Structural Representations: Bond-Line Notation
- Page ID
- 237762
As stated previously, organic chemists use several different notations to represent organic molecules. While a molecular formula only indicates the types and quantities of elements that are present in a molecule, nearly all of the compositional characteristics of an organic substance can be determined by analyzing a structure that is symbolized using expanded structural notation. The types of elements that are present are indicated by the elemental symbols that are shown in the corresponding structural representation. The quantity of each element, as well as the total number of bonds and lone pairs that are present in a molecule, can be established by counting the number of times that each symbol is written in a molecular image. Furthermore, the connectivity, or relative bonding arrangement, of each atom, bond, and lone pair can be explained by describing the placement of each of these components, relative to one another, in an organic structural representation. The types of bonds that are found in a molecule can be determined by counting the number of lines that are drawn between any two atoms in the corresponding image. Structures that are drawn using Valence Shell Electron Pair Repulsion notation also reflect the three-dimensional spatial orientations of organic molecules and their constituent atoms, in addition to all of the compositional characteristics that are listed above above. However, since organic molecules can contain thousands of individual carbon atoms, the expanded and VSEPR images of these substances can become prohibitively-large in size. Furthermore, representing large molecules using these notations is incredibly time-consuming, due to the amount of detail that must be incorporated into the pictures that are generated. Therefore, in order to visually-represent organic molecules using smaller images, organic chemists have symbolized these substances using condensed structural notation, which eliminates specific structural components from a provided expanded structure or VSEPR picture and, instead, uses a "formula-style" format to indicate the number of hydrogens that are bonded to a particular non-metal. A second, more minimalistic, drawing convention, which is known as bond-line notation, will be discussed in the following paragraphs.
Images that are symbolized using bond-line notation, which uses "zig-zag" lines to represent the bonds that exist between consecutive carbon atoms, are generated by eliminating specific structural components from a provided expanded, VSEPR, or condensed picture. Therefore, bond-line structures are substantially smaller in scale, compared to the structures from which they are derived. In particular,
- all lone pairs,
- all non-metal/hydrogen bonds,
- all elemental symbols that represent carbon, C, and
- any hydrogen, H, elemental symbols that are associated with carbon atoms
should be removed when generating a bond-line structure from an image that is drawn using a more detailed notation. All remaining single bonds should be represented using "zig-zag" lines that are analogous to those that are used in VSEPR notation. This "zig-zag" pattern can be initiated using either an upward or a downward angle, because, as stated previously, the second atom in any organic structure can be bonded to any "side" of the first elemental symbol that is written. Any double or triple bonds that are present in a molecule should be symbolized by writing an extra "floating" line in the corresponding position in the "zig-zag" pattern that has been drawn. Furthermore, any hydrogen atoms that are bonded to heteroatoms should be retained and represented using the condensed "JHQ" format that was introduced in the previous section of this chapter. Recall that the generic placeholder letter "J" should be replaced with the elemental symbol of a non-metal and that the "Q" subscript corresponds to the number of hydrogens that are bonded to that atom. If only a single hydrogen is associated with a particular atom, no subscript should be written, since values of "1" are usually implicitly-understood in chemistry.
For example, represent each of the following organic molecular structures using bond-line notation.
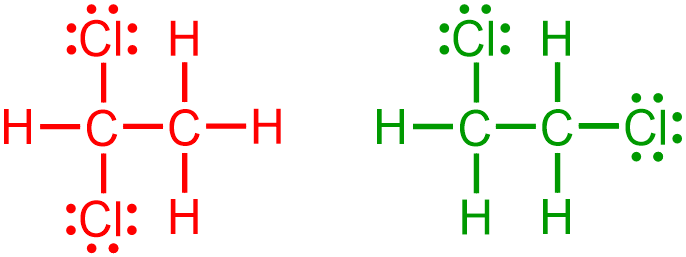
A bond-line structure is generated by eliminating specific structural components from a provided expanded, VSEPR, or condensed picture. In particular, when generating a bond-line structure from an image that is represented in a more detailed notation, all lone pairs,
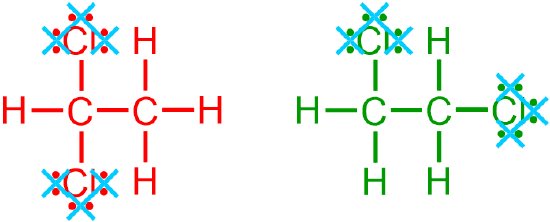
all non-metal/hydrogen bonds,
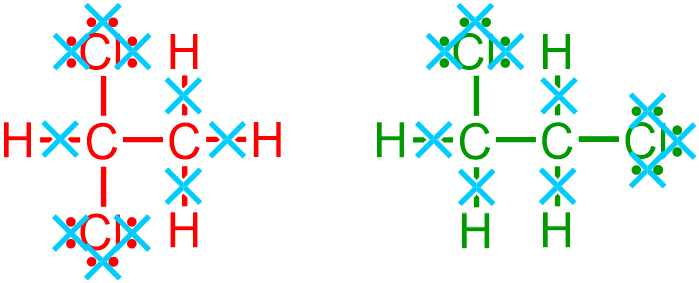
all elemental symbols that represent carbon, C,
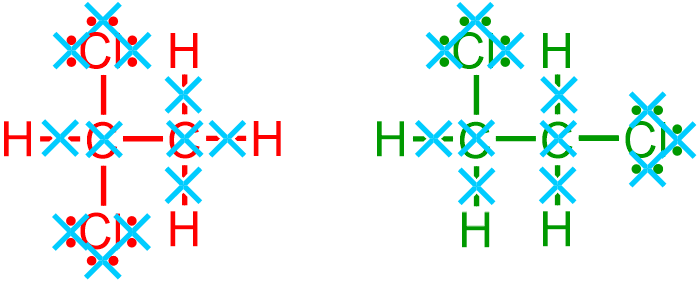
and any hydrogen, H, elemental symbols that are associated with carbon atoms
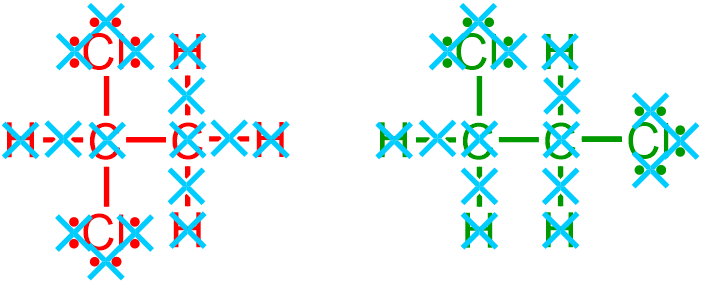
are removed, as indicated by the light blue " " symbols in the images that are shown above. After eliminating all of these structural characteristics from the given molecule, the following elemental symbols and bonds remain.
" symbols in the images that are shown above. After eliminating all of these structural characteristics from the given molecule, the following elemental symbols and bonds remain.

In a bond-line structure, these remaining single bonds are represented using "zig-zag" lines that are analogous to those that are used in VSEPR notation. This "zig-zag" pattern can be initiated using either an upward or a downward angle, because, as stated previously, the second atom in any organic structure can be bonded to any "side" of the first elemental symbol that is written. Therefore, both red structures that are shown below are equivalent to one another, as are both green images. These pictures, which are substantially smaller in scale, relative to the expanded structures from which they were derived, are both chemically-correct bond-line representations of the given images.
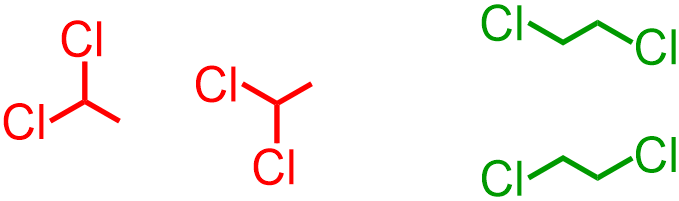
Although bond-line structures are significantly more challenging to interpret, relative to expanded, VSEPR, and condensed representations, nearly all of the compositional characteristics of a molecule can still be determined by analyzing a structure that is symbolized using bond-line notation. The types and quantities of heteroatoms that are present in a molecule can be determined by counting the number of times that each elemental symbol is written in a molecular image. In bond-line notation, a carbon atom is understood to be located at every point at which two or more bonds meet and on the end of every line that is not explicitly-associated with a heteroatom. Furthermore, each carbon atom is assumed to be bonded to enough hydrogens to achieve its preferred four-bond configuration. The total number of bonds and lone pairs that are present in a molecule, as well as the connectivity of each atom, bond, and lone pair, can be inferred by applying an understanding of standard non-metal bonding configurations. Finally, the types of bonds that are found in a molecule can be determined by counting the number of lines that are drawn between any two atoms in the corresponding bond-line image. However, the bond-line images that are drawn above are two-dimensional drawing conventions and, therefore, do not correctly depict the spatial orientations of organic molecules or their constituent atoms.

