10.1: Organic Chemistry: Introduction and Review
- Page ID
- 237746
The previous chapters of this textbook have explored different general chemistry topics, including fundamental chemical concepts, theories, and quantities that were subsequently applied to derive important mathematical relationships and determine the properties and reactivity of all of the elements that are represented on the periodic table. In contrast, the current chapter will discuss several topics that are specific to organic chemistry, which is defined as the study of carbon-containing compounds.
Carbon is one of the most abundant elements on Earth and, more importantly, is extremely versatile. Atoms of carbon can form four stable covalent bonds with each other and with many other elements and, consequently, are found in millions of different substances. In particular, most living organisms are primarily composed of organic molecules, which are covalently-bonded chemicals that must be comprised of carbon, C, usually contain hydrogen, H, and can, more rarely, include other non-metals. Because nitrogen, N, oxygen, O, phosphorus, P, sulfur, S, and the halogens, X, are less prevalent in organic molecules, relative to carbon and hydrogen, these eight non-metals, which are all located on the right side of the periodic table, are collectively-known as heteroatoms. Metalloids, such as boron, B, and silicon, Si, are generally not found in organic compounds. Furthermore, since the structures of organic molecules must, by definition, be covalently-bonded, metals and polyatomic ions, which can only bond through ionic interactions, cannot be incorporated into organic materials.
As stated above, millions of molecules contain carbon and, therefore, can be classified as organic. However, many organic materials have distinct characteristics and, consequently, can be further categorized according to the specific elemental composition of, and types of bonds that are present in, their corresponding structures. Hydrocarbons are defined as organic molecules that contain only carbon, C, and hydrogen, H. Because these substances are comprised of only two elements, a relatively small percentage of organic molecules can be classified as hydrocarbons. Additionally, recall that covalent molecules can contain single bonds, double bonds, or triple bonds, which are produced when one, two, or three pairs of electrons, respectively, are shared between the same set of atoms. Because the presence of a double or a triple bond in a structure changes the reactivity of the corresponding molecule, organic molecules that contain these multiple bonds are classified using different terminology, relative to those substances that are solely-comprised of single bonds. Alkanes, alkenes, and alkynes are defined as hydrocarbons that contain only single bonds, one or more double bonds, and one or more triple bonds, respectively. Two-carbon alkane, alkene, and alkyne structures are shown below in the first, second, and third images, respectively, in Figure \(\PageIndex{1}\). Each carbon that is present in these structures is associated with four total bonds, as is typical of organic molecules. However, as the number of bonds that exist between the carbon atoms increases, the relative number of hydrogens that are bonded to each carbon and, therefore, that are found in each molecule, decreases. As a result, alkanes are classified as saturated, because their corresponding structures contain the maximum possible number of hydrogens, and alkenes and alkynes, which contain fewer hydrogens, are categorized as unsaturated. In Chapter 7, these terms were analogously used to describe the amount of solute that is present in a solution, relative to a calculated maximum quantity.

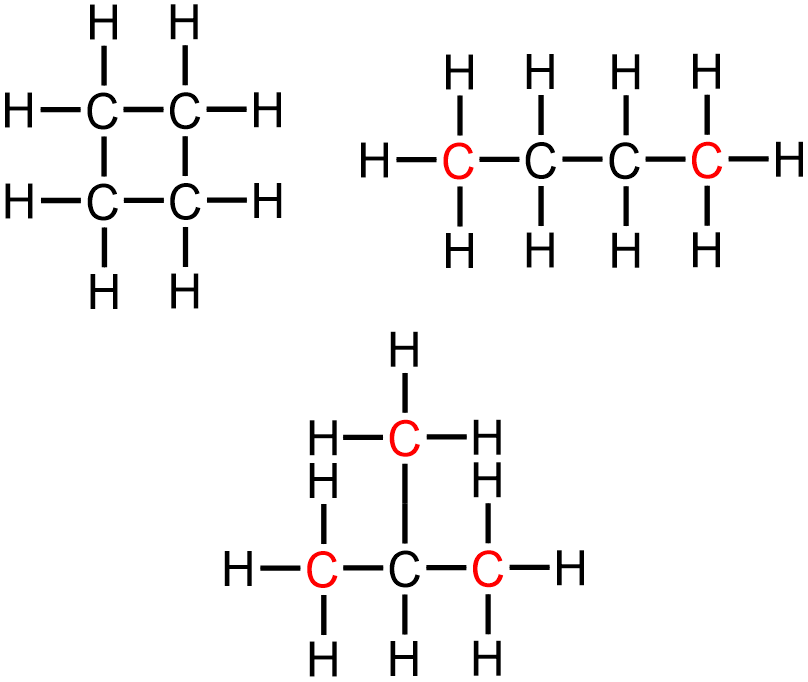
Finally, each of these types of hydrocarbons can be categorized according to their connectivity, which indicates the relative bonding arrangement of the carbon atoms that are contained in the structure. The carbon atoms that are present in a cyclic structure, which often resembles a geometric shape, must connect with one another to form a "ring" that has no discernible "ends." In contrast, an acyclic structure, which can be can be further classified as being linear or branched, cannot contain a "ring." Instead, the atoms that are found within this type of molecule must be connected to one another in a "chain" that has at least two distinctive "ends." In order to be categorized as a linear structure, all of the carbon atoms that are present must be organized in a single "chain" that has exactly two discernible "ends," and a branched structure must contain more than two unique "ends." The top-left, top-right, and bottom images in Figure \(\PageIndex{2}\) show four-carbon alkanes that are connected as cyclic, linear, and branched structures, respectively, and the "end," or terminal, carbons that are present in each structure are indicated in red.
The remaining sections of this chapter will further investigate the structures and reactivity of alkanes, which, due to their limited elemental composition and lack of multiple bonds, can be discussed more comprehensively than other types of organic molecules. Because the structures of alkanes are relatively similar to one another, these chemicals all share several common physical properties, which, as stated in Chapter 3, correspond to the observable, descriptive characteristics of a substance. As stated in Chapter 7, the electron distributions of alkanes and water are dissimilar, and, consequently, these types of chemicals are immiscible, or insoluble, in one another. As a result, a heterogeneous bilayer, in which organic alkanes and water are present as two visually-distinctive liquids, forms when these chemicals are mixed together. Furthermore, because most alkanes have density values that are less than 1.00 g/mL, which is the density of liquid water, these organic substances will be present in the top, or floating, layer in a heterogeneous mixture, as shown below in Figure \(\PageIndex{3}\). Finally, since all hydrocarbons must, by definition, contain carbon, these types of organic materials have similar electron distributions, relative to each other, and, therefore, also do not readily dissolve in water. As a result, liquid alkanes are often used as solvents in which other water-insoluble organic molecules can be dissolved.


