10.4: Organic Structural Representations: VSEPR Notation
- Page ID
- 237749
As stated previously, organic chemists use several different notations to represent organic molecules. While a molecular formula only indicates the types and quantities of elements that are present in a molecule, nearly all of the compositional characteristics of an organic substance can be determined by analyzing a structure that is symbolized using expanded structural notation. The types of elements that are present are indicated by the elemental symbols that are shown in the corresponding structural representation. The quantity of each element, as well as the total number of bonds and lone pairs that are present in a molecule, can be established by counting the number of times that each symbol is written in a molecular image. Additionally, the connectivity, or relative bonding arrangement, of each atom, bond, and lone pair can be explained by describing the placement of each of these components, relative to one another, in an organic structural representation. Finally, the types of bonds that are found in a molecule can be determined by counting the number of lines that are drawn between any two atoms in the corresponding image. However, because the world is filled with three-dimensional objects, structures that are drawn using expanded structural notation, which is used to generate two-dimensional pictures, do not correctly depict the spatial orientations of organic molecules or their constituent atoms.
Covalent molecules are formed when the unpaired valence shell electrons found within two atoms interact to form shared pairs of electrons. Because electrons are negatively-charged subatomic particles, the presence of multiple electrons within the bonds and lone pairs of a molecule is moderately destabilizing, as identically-charged particles repel one another when in close proximity. Consequently, increasing the distance between these bonds and lone pairs will minimize the effects of these repulsive forces and improve the overall stability of the corresponding molecule. Representing an organic molecule using expanded structural notation, which is a two-dimensional drawing convention, inherently restricts the degree to which electron pairs can be separated. Valence Shell Electron Pair Repulsion, or VSEPR, notation does not have any spatial restrictions and, therefore, can be utilized to depict molecules using three-dimensional structures. For this reason, VSEPR pictures more accurately represent the true shapes of organic molecules, relative to expanded structural notation, as three-dimensionally distributing the bonds and lone pairs that are present in a molecule maximizes their physical separation.
In order to represent the three-dimensional directions in which bonds can be oriented within covalent molecules, VSEPR notation uses three distinctive symbols, which are shown below in Figure \(\PageIndex{1}\). The left-most symbol in Figure \(\PageIndex{1}\) is the line that has primarily been used to represent covalent bonds in the previous sections of this textbook. This symbol, which is known as a stick bond, does not convey any three-dimensional information. The middle image in Figure \(\PageIndex{1}\), which is called a wedged bond, indicates that the atom that is located on the wider end of the symbol is oriented toward the viewer, relative to the atom that is found on the pointed end of the bond. The right-most image in Figure \(\PageIndex{1}\), which is known as a dashed bond, denotes that the atom lthat is ocated on the wider end of the symbol is oriented away from the viewer, relative to the atom that is found on the pointed end of the bond.

As stated previously, atoms of carbon can form four covalent bonds and do not bear any lone pairs when incorporated into organic molecules. This type of bonding configuration is represented in three dimensions using a tetrahedral VSEPR shape, in which the carbon atom is associated with two stick bonds, one wedged bond, and one dashed bond. When connecting atoms using VSEPR notation, stick bonds should typically be arranged in a left-to-right "zig-zag" pattern, and the wedged and dashed bonds that are associated with a particular atom should both be positioned on the same side of the elemental symbol that is shown. Specifically, if the stick bond to the left of a given atom "zigs up" to connect with that atom, the wedged and dashed bonds on that atom should both be angled upward, and if the stick bond to the left of an atom "zags down," the wedged and dashed bonds that are associated with that atom should both be angled downward. Furthermore, a wedged bond should always be written to the left of the corresponding dashed bond, as shown below in Figure \(\PageIndex{2}\). When using VSEPR notation to represent an organic molecule, the generic placeholder letters, "Z," that are shown in these images are replaced with the symbols of the elements that are bonded to the carbon atom.
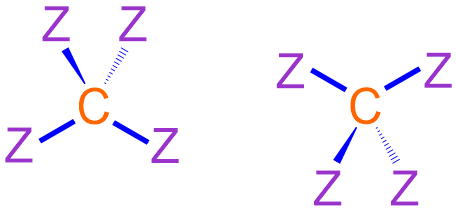
Because all organic molecules must contain carbon, C, this element should be written first when symbolizing an organic substance using VSEPR notation. In order to represent the bond that exists between consecutive atoms, a line that connects their corresponding elemental symbols should be drawn. When connecting atoms using VSEPR notation, the second carbon can be bonded to any "side" of the first elemental symbol that is written, but the connectivity of all subsequent carbon atoms must be considered relative to those that are already present. However, if a VSEPR structure is generated from a provided image, in which an organic molecule is symbolized using an alternative notation, the relative placement of each atom must remain consistent between both representations. Specifically, bonds that are written in a horizontal, or left-to-right, direction in expanded structural notation become stick bonds when representing that molecule using VSEPR notation. Lines that are oriented straight upward and straight downward when drawing a molecule using expanded structural notation translate to wedged bonds and dashed bonds, respectively, in a VSEPR structure. Finally, the correct number of lone pairs should be added to any heteroatom symbols. As explained in the previous section, nitrogen, N, and phosphorus, P, will each bear one lone pair when incorporated into a molecule that is represented using VSEPR notation. Oxygen, O, and sulfur, S, will each be associated with two lone pairs, and three lone pairs must be drawn on the elemental symbol of a halogen, X, that is present in an organic molecule.
For example, represent each of the following organic molecular structures using VSEPR notation.
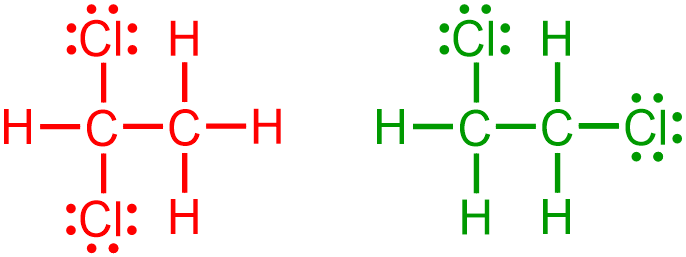
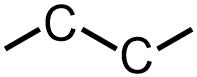
Because all organic molecules must contain carbon, C, the two carbon atoms that are represented in these molecules are written first in the VSEPR structure that is being developed. Because the bonds that connect these carbons are written in a horizontal, or left-to-right, direction in each of the given expanded structures, these bonds become "zig-zag" stick bonds in the corresponding VSEPR images. Because, as stated above, the second carbon can be bonded to any "side" of the first elemental symbol that is written, both of the images that are shown below are chemically-equivalent to one another.

Since both of the carbon atoms in the given structures are horizontally-bonded to one additional atom, a second stick bond should "zig," or "zag," relative to each "C" in the structure that is being developed. The following image results upon modifying the first structure that is shown above to reflect these additional bonds.
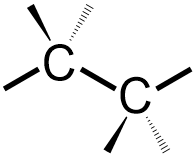
Because two stick bonds are now associated with the elemental symbols that are shown in the picture that is drawn above, one wedged bond and one dashed bond must be added to each of these "C"s. The wedged and dashed bonds that are associated with a particular atom should both be positioned on the same side of the elemental symbol that is shown, and a wedged bond should always be written to the left of the corresponding dashed bond. Specifically, since the stick bond to the left of the first carbon atom in the image shown above "zigs up" to connect with that carbon, the wedged and dashed bonds on that atom are both angled upward on that carbon in the structure that is being developed. Additionally, because the stick bond to the left of the second carbon atom "zags down," the wedged and dashed bonds that are associated with that atom are both angled downward on the corresponding VSEPR carbon, as shown below.
Since these VSEPR structures are being developed from provided images, the relative placement of each atom must remain consistent between both representations. The left-most carbon atom in the first given structure is horizontally-bonded to a hydrogen and vertically-bonded to two chlorine atoms. Therefore, in the corresponding VSEPR structure, the elemental symbol for hydrogen, H, should be placed on the stick bond that is associated with the left-most carbon atom, and the elemental symbol for chlorine, Cl, should be written on the wedged and dashed bonds. Furthermore, since the right-most carbon atom in the first given structure is bonded to three hydrogens, the elemental symbol for hydrogen, H, should be placed on all three of the bonds in the VSEPR structure that is being developed, as shown below.

The first carbon atom in the second given structure is horizontally-bonded to a hydrogen, vertically-bonded to a chlorine atom that is oriented straight upward, and vertically-bonded to a hydrogen atom that is pointed straight downward. Therefore, in the corresponding VSEPR structure, the elemental symbol for hydrogen, H, should be placed on the stick bond and the dashed bond that is associated with the left-most carbon atom in the corresponding VSEPR image, and the elemental symbol for chlorine, Cl, should be written on the wedged bond. Additionally, because the right-most carbon atom in the second given structure is horizontally-bonded to a chlorine and vertically-bonded to two hydrogen atoms, the elemental symbol for chlorine, Cl, should be placed on the stick bond that is associated with the second carbon atom, and the elemental symbol for hydrogen, H, should be written on the wedged and dashed bonds in the VSEPR structure that is being developed, as shown below.
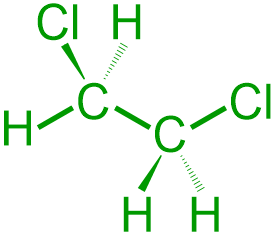
Finally, the images that are shown above are completed by adding three lone pairs to the elemental symbol of each chlorine. The following pictures are both chemically-correct VSEPR representations of the given expanded structures.
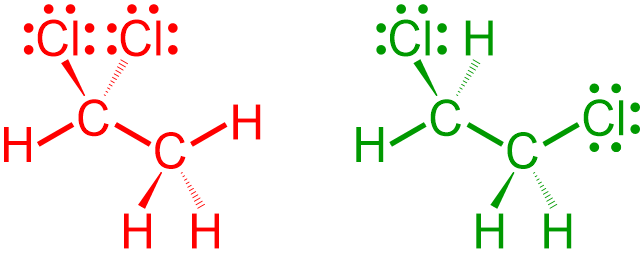
All of the compositional characteristics of a molecule can be determined by analyzing a structure that is symbolized using VSEPR notation. The types of elements that are present are indicated by the elemental symbols that are shown in the corresponding structural representation. The quantity of each element, as well as the total number of bonds and lone pairs that are present in a molecule, can be established by counting the number of times that each symbol is written in a molecular image. Additionally, the three-dimensional connectivity of each atom, bond, and lone pair can be explained by describing the relative placement of, and the VSEPR bond symbolism associated with, each of these components. Finally, the types of bonds that are found in a molecule can be determined by counting the number of lines that are drawn between any two atoms in the corresponding VSEPR image.

