3.19: Covalent Bonding: Expanded Valence Exceptions to the Octet Rule
- Page ID
- 215887
Recall that covalent bonds are produced when unpaired electrons found within two atoms, which must be classified as either non-metals or metalloids, interact to form a shared pair of electrons. The previous section discussed hydrogen and boron, which, due to inherent deficiencies in their atomic electron configurations, are unable to achieve octet configurations when bonding. Nevertheless, these elements can still pair their electrons with those found in other non-metals, in order to produce stable, but electron-deficient, covalent molecules. Carbon, nitrogen, oxygen, and fluorine strictly adhere to the "octet rule," as these elements will always bond so as to be associated with eight, fully-paired electrons, without exception. All of non-metals and metalloids that are not explicitly listed above are able to achieve octets when bonding, but can also deviate from the "octet rule" by expanding their valences to accommodate more than eight electrons. The information in the following paragraphs will explain these electronic variations and their impact on the bonding patterns of these elements. Finally, since these elements can still form stable covalent molecules by pairing their electrons with those found in other non-metals, Lewis structures for the resultant covalent molecules will be provided and used to derive corresponding chemical formulas and chemical names.
Elements that Can Achieve Expanded Valences When Bonding
Recall that electrons are located at defined distances from the nucleus, which are known as energy levels, and that each of the seven periods on the periodic table has a corresponding energy level. Furthermore, every energy level is associated with one or more orbitals, which are classified according to their shapes, s, p, d, and f. Each of the seven energy levels is associated with a single s orbital. While the first energy level does not have any corresponding p orbitals, each of the remaining energy levels contain three p orbitals that are distinguished based on their defined orientation in three-dimensional space. Finally, remember that every orbital can contain a maximum of two electrons. As a result, every energy level can hold two electrons in its s orbital. Furthermore, since three unique p orbital orientations exist, beginning at the second energy level, each of the elements found within the second, third, fourth, fifth, sixth, or seventh periods of the periodic table can hold an additional six electrons in its combined p orbitals. Therefore, by completely filling the s and p orbitals of its valence shell, any atom that located in one of these periods can hold a total of eight electrons and achieve an octet configuration when bonding.
d orbitals are first found within atoms located in the third period of the periodic table. These orbitals are typically partially or completely unoccupied in atomic electron configurations, as they are higher in energy than and, therefore, less stable relative to, the s and p orbitals found at the same energy level. However, the presence of these additional orbitals causes the elements that contain them to bond in atypical ways. The specific deviation that manifests is based on both the type of bond that is formed and the metallic classification of the element.
Transition metals, which are only able to form ionic bonds and are classified as metals, by definition, were discussed in Section 3.8. Recall that these elements ionize in unique ways, due to the d orbitals that they contain. In particular, transition metals are able lose inner shell electrons, in addition to their valence electrons, and can lose more than three total electrons, which is energetically-unfavorable for main group metals. Finally, most transition metals are able to achieve stable electron configurations through multiple ionization pathways. For example, iron (Fe) can lose two electrons to produce an ion symbolized as Fe+2. However, iron is equally likely to ionize by losing three electrons, resulting in the formation of an Fe+3 cation. The presence of d orbitals does not impact the ionization of non-metals.
Lewis Structures of Covalent Molecules that Contain Atoms with Expanded Valences
d orbitals can, however, impact the covalent interactions of non-metals and metalloids. Recall that electron dot structures visually represent an atom's valence electrons as dots that are placed on one of four "sides" of an elemental symbol. As stated above, beginning in the second period of the periodic table, every atom contains one s orbital and three p orbitals in its valence shell. Therefore, the number of "sides" in an electron dot structure directly corresponds to the total number of s and p orbitals that exist within an atom. Carbon, nitrogen, oxygen, and fluorine are all found in the second period of the periodic table and, consequently, only contain s and p orbitals. As a result, these elements strictly adhere to the "octet rule," as they can be associated with a maximum of eight, fully-paired electrons when bonding, without exception.
By correctly applying the rules provided in Section 2.7 for placing the dots around an elemental symbol, electron pairs will naturally exist within any atom that contains more than four valence electrons. Maximizing the number of paired electrons within an atom is favorable, because, as has been stated several times, electrons are most stable when they exist in pairs. However, the presence of d orbitals within an atom can cause one or more of its electron pairs to separate or "unpair." Figure \(\PageIndex{1}\) shows three possible electron dot structures for sulfur, which contains six valence electrons. Furthermore, sulfur is found in the third period of the periodic table and, therefore, contains d orbitals. The left-most structure represents sulfur's most stable electron configuration, as it contains two pairs of electrons. The middle and right-most structures are generated by separating one or both of these electron pairs, respectively.

Recall that covalent bonds are produced when unpaired electrons found within two atoms interact to form a shared pair of electrons. Therefore, a sulfur atom that exists in its most stable configuration, as shown in the left-most structure in Figure \(\PageIndex{1}\), can form two covalent bonds, as it contains two unpaired electrons. If one of sulfur's electron pairs is separated, as illustrated in the middle structure in Figure \(\PageIndex{1}\), four covalent bonds can be created. Finally, if both of sulfur's electron pairs are "unpaired," as shown in the right-most structure in Figure \(\PageIndex{1}\), the resultant covalent molecule will contain six bonds. A chemically-correct Lewis structure that represents the covalent molecule that forms upon combining each of these sulfur configurations with fluorine, which is found in the second period of the periodic table and contains 7 valence electrons, is shown below in Figure \(\PageIndex{2}\).
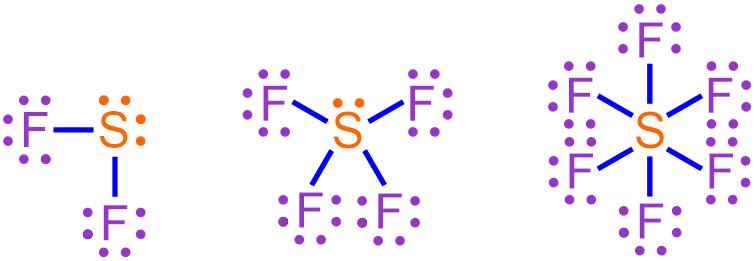
Figure \(\PageIndex{2}\): Lewis structures for the covalent molecules that are formed upon combining fluorine with each of the sulfur configurations shown in Figure \(\PageIndex{1}\).
Since all of the electrons shown above are paired, each of these structures represents the most stable bonding arrangement that can be achieved by combining fluorine with the corresponding sulfur configuration shown in Figure \(\PageIndex{1}\). Each line in a Lewis structure represents a covalent bond, or a shared pair of electrons. Therefore, the sulfur atoms shown above in Figure \(\PageIndex{2}\) have achieved octet, decet, and dodecet configurations, as they are associated with 8, 10, and 12 electrons, respectively. The left-most Lewis structure in Figure \(\PageIndex{2}\) was first seen in Section 3.15, which presented the process for drawing Lewis structures of covalent molecules that bonded according to the octet rule. The covalent molecules represented by the middle and right-most Lewis structures in Figure \(\PageIndex{2}\) are hypervalent molecules, as they contain atoms with expanded octets.
Unfortunately, predicting the atomic electron configuration with which sulfur will bond is impossible, as each of the structures in Figure \(\PageIndex{1}\) are relatively stable. Furthermore, all of the metalloids and non-metals that are located in the third, fourth, fifth, sixth, and seventh periods of the periodic table contain d orbitals and, therefore, can bond using either "paired" or "unpaired" atomic electron configurations. As a result, students enrolled in this course will not be expected to generate Lewis structures for molecules that contain atoms with expanded valences. Instead, Lewis structures for hypervalent covalent molecules will be provided and used to derive corresponding chemical formulas and chemical names.
Writing Chemical Formulas of Covalent Molecules that Contain Atoms with Expanded Valences
The chemical formula of a covalent molecule that contains a hypervalent atom can be determined using the rules presented in Section 3.16. For example, consider the Lewis structure shown below, which represents a hypervalent covalent molecule that is formed when fluorine and sulfur bond with one another.
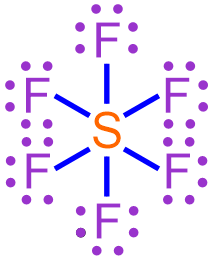
For a covalent molecule, the information represented in its chemical formula must be a direct reflection of its Lewis structure. Elemental symbols are incorporated into a chemical formula by counting the number of times that each symbol appears in the corresponding Lewis structure. In order to ensure consistent formatting, the elemental symbol that appears fewer times is written first in a covalent chemical formula, and subscripts are used to indicate how many times each elemental symbol appears in the Lewis structure. As indicated previously, values of "1" are usually implicitly-understood in chemistry and, therefore, should not be written in a chemical formula. The subscripts must not be reduced to the lowest-common ratio of whole numbers, even if it is mathematically-possible to do so, as dividing the subscripts would cause their values to be inconsistent with the number of times that each elemental symbol appears in the Lewis structure.
The Lewis structure shown above contains six fluorine atoms and one sulfur atom. As there are fewer sulfur atoms in the Lewis structure, the elemental symbol "S" is written first in the corresponding chemical formula, and fluorine's elemental symbol, "F," is written second. No subscript should be written on sulfur's elemental symbol, as values of "1" should not be explicitly-written in a chemical formula. A subscript of "6" should be written on fluorine's elemental symbol. The resultant chemical formula, SF6, accurately summarizes the information in the Lewis structure shown above and, therefore, is the chemically-correct formula for this covalent molecule.
Naming Covalent Molecules that Contain Atoms with Expanded Valences
The chemical name of a covalent molecule that contains a hypervalent atom can be derived using the information outlined in Section 3.17.
For a covalent molecule, the information represented in its chemical name must also be a direct reflection of its Lewis structure. Therefore, the chemical name of a covalent molecule must not only contain information that indicates the identities of its constituent elements, but also must reflect how many of each of those elements are present within the molecule. Note that, if written properly, the chemical formula for a covalent molecule also contains this information and, therefore, can be used as the basis for developing a chemical name. Elemental names are incorporated into a covalent molecule's chemical name in the order in which their corresponding elemental symbols appear in the chemical formula. The suffix on the second elemental term is replaced with "-ide," in order to indicate its secondary placement within the chemical formula. Finally, since the subscripts in a covalent chemical formula are used to indicate how many times each elemental symbol appears in the molecule's Lewis structure, corresponding numerical prefixes must be incorporated into the molecule's chemical name. Remember that the prefix "mono-" is never used to change the first elemental term in a covalent chemical name and should only be used as a modifier on the remaining term if the secondary element is oxygen. Finally, an "a" or "o" at the end of a prefix is usually dropped if the name of the element that is being altered begins with a vowel.
Since the elemental symbol "S" appears first in the chemical formula that was developed above, "sulfur" is the basis of the first word in the molecule's chemical name. The subscript on this elemental symbol, an unwritten "1," corresponds to prefix of "mono-." However, this prefix is not used to alter the first elemental term in a covalent chemical name. Therefore, the first word in the chemical name of this molecule is "sulfur."
Because the elemental symbol "F" is written second in the chemical formula that was developed above, "fluoride" becomes the basis of the second word in the molecule's chemical name. The suffix on this elemental term is "-ide," as an indicator of its secondary placement within the chemical formula. The subscript on this elemental symbol, a "6," corresponds to prefix of "hexa-." Therefore, the second word in the chemical name of this covalent molecule is "hexafluoride."
The result of combining these words, "sulfur hexafluoride," is the chemically-correct name for SF6.

