3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges
- Page ID
- 214227
In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, were described, and the charges of several resultant ions were determined. Unfortunately, these processes were quite lengthy. First, the number of valence electrons possessed by the initial neutral atom was established. Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. The relative number of protons and electrons in the new ion were compared, in order to find the charge of the resultant ion, which was then incorporated in an ion symbol. Finally, a new ion name was presented. Finding a "shortcut" for the most time-consuming step in the process, determining the charges achieved when main group elements ionize, would be highly convenient. A pattern-based "charge shortcut" does, indeed, exist, in the form of a trend that spans the main group or "A-Block" columns on the periodic table.
Recall that all elements found within the same column on the periodic table have the same number of valence electrons. As establishing the number of valence electrons within the initial atom is the first step in the processes described above, the analysis of all elements in the same group will begin identically. Furthermore, since all subsequent procedural steps are dependent on that initial valence electron count, all elements in the same group will gain or lose the same number of electrons to achieve an octet configuration. Consequently, all elements in the same group will form ions with the same charge.
Figure \(\PageIndex{1}\) shows the charge pattern for main group element ionization. The groups marked with an "X" do not contain main group elements that ionize. Recall that the noble gases, the elements found in Group 18 or 8A, are naturally stable, because they inherently possess an octet of valence electrons. Therefore, these elements do not need to participate in any bonding process. The elements in Group 14, or 4A, only have four valence electrons in their atomic form, requiring that they either gain four additional valence electrons or lose their pre-existing four valence electrons, in order to achieve an octet configuration. However, gaining or losing more than three valence electrons is energetically-unfavorable and will not occur. Therefore, these elements are energetically-disqualified from ionizing.
The remaining columns each have an associated positive or negative numerical value that indicates the charge that results when elements in that column are ionized. For example, consider iodine (I). Because this element is located in Group 17, or 7A, on the periodic table, it will ionize to form an anion with a –1 charge. Therefore, the resultant ion is symbolized as I–1 and is named the iodide ion. Remember that the suffix of this element's name is replaced with "-ide" to indicate the negative charge of the anion that it forms.
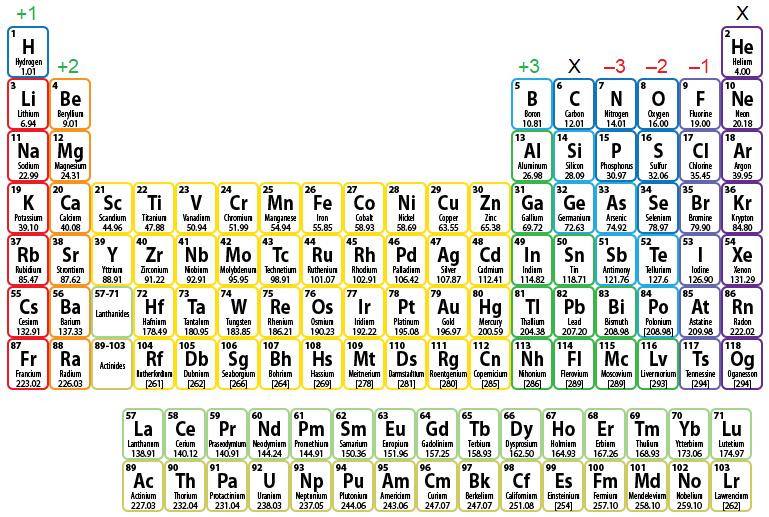
Figure \(\PageIndex{1}\): Charge Pattern for Main Group Element Ionization.
Finally, note that this charge pattern only applies to main group element ionization. As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12, do not have predictable reactivity patterns and trends. As a result, determining how these elements ionize is relatively complex and will not be discussed until a later section in this chapter.

