2.7: Applications of Electron Configurations: Valence Electrons and Electron Dot Structures
- Page ID
- 214189
Valence Electrons
As was mentioned in a previous section of this chapter, electrons are highly important, because a specific subset of electrons, called valence electrons, are solely-responsible for determining how elements bond with one another. The number of valence electrons that are present in an atom can be determined from that atom's electron configuration. Valence electrons are found in the orbitals associated with an atom's highest occupied energy level. The remaining electrons, which are called inner shell electrons, do not participate in bonding and are, therefore, not important to study.
Consider sulfur's electron configuration, which was determined in the previous section and is replicated below.
1s22s22p63s23p4
Recall that the energy levels in an electron configuration are the leading red numbers that denote the start of a new energy level/orbital combination. Sulfur has electrons in the first, second, and third energy levels, as indicated by the leading red 1, 2's, and 3's, respectively. Valence electrons are those found in the highest occupied energy level. Therefore, in this case, only those electrons associated with an energy level/orbital combination beginning with a 3 need to be considered. Since two energy level/orbital combinations begin with a 3, both orbitals are selected for further consideration:
3s23p4
The superscripts associated with these orbitals total to 6. Therefore, sulfur has 6 valence electrons.
While an electron configuration represents all of the electrons present in an atom of an element, chemists are only truly interested in an atom's valence electrons, since, as indicated above, those are the electrons that are solely-responsible for determining how elements bond with one another. Therefore, finding a "shortcut" for determining how many valence electrons are present in an atom would be highly convenient. Such a "shortcut" does, indeed, exist. In a previous section of this chapter, three systems for labeling the groups, or columns, on the periodic table were presented. The second system, which is called the "A/B System," was indicated to provide insight into the electronic character of elements found within that group.
Again, consider sulfur, S, which, based on its electron configuration, has 6 valence electrons.
Sulfur is located in the 16th column of the periodic table. However, the "A/B System" is used to label the main group elements. Group 16 is the 6th column in the main group, or "A-Block," columns of the periodic table and so is labeled as Group 6A. Note that sulfur's valence electron count matches its group number in the "A/B System." This connection applies to nearly all elements found in the main group columns of the periodic table. Helium is the only exception to this rule, as it is found in Group 8A, but only contains two total electrons. This inconsistency invalidates the "A/B shortcut" method, and the electron configuration method must be employed to determine that both of helium's electrons are valence electrons.
Since the "A/B System" group number corresponds to the number of valence electrons that are present in an atom, all elements found within the same column have the same number of valence electrons. Since an atom's valence electrons are solely-responsible for determining how elements bond with one another, this commonality in electronic character explains why all of the elements within the same group share similar properties.
Electron Dot Structures
Electron dot structures surround the elemental symbol of an element with one dot for every valence electron that the element contains. When drawing an electron dot structure, three rules must be followed:
- The first dot can be placed on any "side" of the elemental symbol (top, bottom, left, or right).
- The first four dots must each be placed on their own "side" of the elemental symbol. In other words, if the first dot is placed on the top of the elemental symbol, the second dot can be placed on the bottom, left, or right of the symbol, but cannot be placed at the top, alongside the first dot.
- The final four dots can again be placed on any "side" of the elemental symbol, but must be arranged such that no more than two dots exist on any "side" of the elemental symbol.
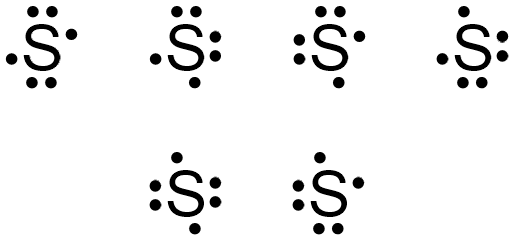
Again, consider sulfur, which has 6 valence electrons.
The elemental symbol for sulfur is S. Since an electron dot structure surrounds an elemental symbol with one dot for every valence electron that the element contains, sulfur's elemental symbol must be surrounded by 6 dots. Based on the rules given above, the dot representing sulfur's first valence electron can be placed on any "side" of the symbol, as shown below in Figure \(\PageIndex{1}\).
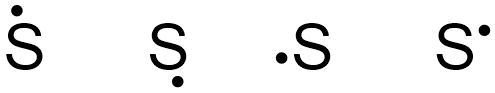
If the first structure in Figure \(\PageIndex{1}\) is chosen as the basis of sulfur's electron dot structure, the dot representing sulfur's second valence electron can be placed on the bottom, left, or right of the elemental symbol, but cannot be placed at the top, alongside the first dot. Figure \(\PageIndex{2}\) shows three structures with acceptable placements for sulfur's first two valence electrons, as well as a structure with an incorrect electron arrangement.
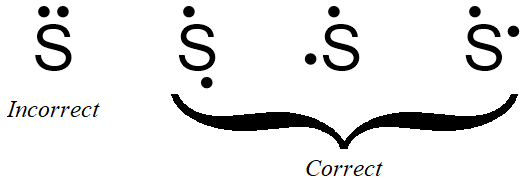
If the final structure in Figure \(\PageIndex{2}\) is chosen as the basis of sulfur's electron dot structure, the dots representing sulfur's third and fourth valence electrons must be placed on the bottom and to the left of the elemental symbol, but cannot be placed at the top or to the right of the elemental symbol. Figure \(\PageIndex{3}\) shows the only structure with an acceptable placement for sulfur's first four valence electrons.
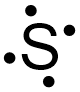
The dots representing sulfur's fifth and sixth valence electrons can again be placed on any "side" of the elemental symbol, but cannot both be placed on the same "side," so that no more than two dots exist on any "side" of the elemental symbol. Figure \(\PageIndex{4}\) shows all of the structures with acceptable placements for sulfur's six valence electrons. Therefore, any of the structures in Figure \(\PageIndex{4}\) is a valid electron dot structure for sulfur.