2.2: The Periodic Table
- Page ID
- 226514
In the 19th century, many previously-unknown elements were discovered, and scientists began to notice that certain sets of elements had similar chemical properties. For example, lithium (Li), sodium (Na), and potassium (K) all form similar compounds upon reaction with an identical secondary element, such as oxygen. In 1869, Dmitri Mendeleev, a Russian chemist, organized all of the currently-known elements according to similarities in their properties. He left gaps in his table for what he thought were undiscovered elements, and he made some bold predictions regarding the properties of those elements. When more elements were found, their properties closely matched Mendeleev’s predictions, and his method for arranging elements gained favor in the scientific community. Because of the regular repetition of certain elemental properties throughout Mendeleev's chart, his organizational system became known as the periodic, or "repeating," table. A modern version of this incredibly important chemical tool is shown below in Figure \(\PageIndex{1}\). The periodic table summarizes a significant amount of information that can be used to locate and categorize elements, as will be described in the following paragraphs.
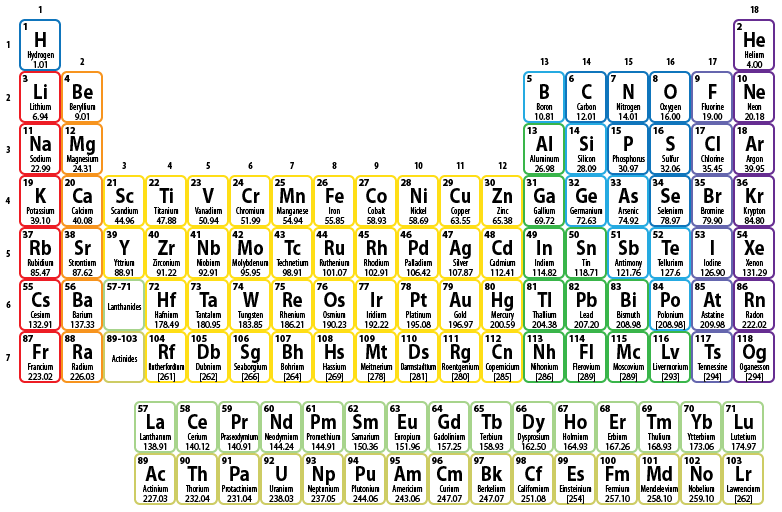
Elemental Names and Symbols
Each box on the periodic table represents a single element. An elemental symbol is always included within each box, in order to indicate which element is located in that position. However, elemental names only appear in some periodic tables. As shown in Figure \(\PageIndex{1}\), the length of some of the elemental names requires that they be printed in a very small font size, which can make them somewhat hard to read. Some periodic tables simply do not incorporate elemental names, as their elimination leaves more room for displaying other information.
Atomic Number
Elements are listed in order of increasing atomic number, which as the number of protons contained within an atom of that element. (The proton is an example of a subatomic particle and will be discussed in greater detail in the following section.) An atomic number is always written above the elemental symbol within each box. Since atomic number increases by one from box to box, no two elements have the same atomic number. Therefore, an atomic number is a unique value that can be used to identify a specific element. For example, carbon (C), and only carbon, has an atomic number of 6. The element with an atomic number of 98 will always be californium (Cf).
Periods
A period is a horizontal (left-to-right) row on the periodic table. Each period is given a numerical value, beginning with "1," which is assigned to the top row. The period number increases by one for every additional row, up to a maximum of 7. It is important to note that the final two "rows" of elements on the periodic table are not periods 8 and 9! Rather, these elements should actually be placed in periods 6 and 7, respectively, based on their atomic numbers. As stated above, atomic number increases by one from box to box within the periodic table. However, the atomic numbers in period 6 seem to jump from 56 to 72. The missing elements, which are assigned atomic numbers ranging from 57 to 71, are moved below the periodic table as a result of their observed properties. Remember that Mendeleev organized elements according to similarities in their properties. Had elements 57 through 71 been inserted into period 6, the remaining elements in this row would no longer align with the elements with which they are most comparable. A similar rationale is applied to the elements that correspond to atomic numbers 89 through 103, which should be placed in period 7.
Groups
A group is a vertical (top-to-bottom) column on the periodic table. Each group is given a numerical value, beginning with "1," which is assigned to the left-most column on the periodic table. The group number increases by one for every additional column, up to a maximum of 18. This method for numbering the groups in the periodic table is known as the "1-18 System." While this numbering convention is widely-used because of its simplicity, it does not give any useful information about the elements found within each column. Two alternative labeling schemes, the "A/B System" and the "Descriptive Naming System," are slightly more challenging to apply than the "1-18 System," but each provides additional insights into the electronic character and properties, respectively, of the elements found within a particular group. The "A/B System" labels each column with both a number (1-10) and a letter (A or B). The "Descriptive Naming System" includes a word or a short phrase that references a fundamental property shared by the elements found within that column. In the sections below, each column will be identified using all three labeling conventions.
Main Group (or Representative) Elements
The elements found in Groups 1, 2, and 13-18 are known as the main group (or representative) elements because they are some of the most common elements found on earth. These elements also have predictable reactivity patterns and trends that can be applied to create straightforward rules that dictate how they interact with other elements. Each of these columns includes an "A" when labeled using the "A/B System." Therefore, the main group elements are known as the "A-Block."
- The left-most column of the periodic table is Group 1 (in the "1-18 System"), or Group 1A (in the "A/B System"). These elements, which are boxed in red in Figure \(\PageIndex{1}\), are known as the alkali metals in the "Descriptive Naming System," because these elements react with water to form hydroxide ions, creating basic, or alkaline, solutions.
- Group 2 is labeled as Group 2A. These elements, which are boxed in orange in Figure \(\PageIndex{1}\), are known as the alkaline earth metals, because these elements also react with water to form basic, or alkaline, solutions. Since the resultant chemicals did not dissolve readily in water, these metals were classified as "earths," which are insoluble solids, in order to distinguish them from the alkali metals.
- Group 13 is labeled as Group 3A and is referred to as the boron group, because boron is the top-most element located in this column.
- Group 14 is labeled as Group 4A and is referred to as the carbon group, because carbon is the top-most element located in this column.
- Group 15 is labeled as Group 5A, and the elements contained in this column are referred to as the pnictogens, which is a term derived from the Greek word "pnigein," which means "to choke," as prolonged exposure to pure nitrogen gas will cause an individual to suffocate.
- Group 16 is labeled as Group 6A, and the elements contained in this column are referred to as the chalcogens, which is derived from the Greek word "chalcos," which means "ore formers," since all of these elements are commonly found in copper ores.
- Group 17 is labeled as Group 7A. Each of the elements found in this column, which are boxed in lavender in Figure \(\PageIndex{1}\), reacts readily with metals to form compounds that can be broadly classified as salts and, therefore, are known as the halogens, which is derived from a combination of Greek words that translate to "salt makers."
- The right-most column on the periodic table, Group 18, is labeled as Group 8A. The elements, which are boxed in purple in Figure \(\PageIndex{1}\), are known as the noble gases, because they do not react readily with other elements and were likened to royals and nobles, who did not often voluntarily interact with "commoners." As a result of their inactivity, these elements exist as single atoms and are called monatomic "compounds."
Transition Metal Elements
The elements found in Groups 3-12 are known as the transition metal elements (or simply as transition metals) because of their location on the periodic table. In order to move from Group 2A to Group 3A, an individual had to move (or transition) through columns 3 through 12. These elements, which are boxed in yellow in Figure \(\PageIndex{1}\), also encompass a broad spectrum of properties that connect (or transition) those found to their left and to their right on the periodic table. Unfortunately, these elements do not have predictable reactivity patterns and trends. As a result, determining how these elements interact with other elements is relatively complex. Each of these columns includes an "B" when labeled using the "A/B System." Therefore, the transition metals are known as the "B-Block."
Metallic Classification
Certain elemental properties span across the periodic table as a whole. One of these characteristics is the metallic classification of an element. A metal is a substance that is shiny, typically silvery in color, and conducts electricity and heat. Metals are malleable, meaning that they can be pounded into thin sheets, and ductile, meaning that they can be drawn into thin wires. A non-metal is typically dull, rather than shiny, and classified as an insulator, which is a substance that is a poor conductor of electricity and heat. Solid nonmetals are very brittle, which means that they will not bend if put under strain, but rather will flake or break. Metalloids have properties between those of metals and non-metals. For example, most metalloids are semi-conductors, which means that they conduct heat and electricity better than non-metals, but not as well as metals.
In Figure \(\PageIndex{1}\), the metalloids are boxed in sky blue. Specifically, boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po) are classified as metalloids. All of the elements to the left of the metalloids, with the exception of hydrogen, are classified as metals. Note that hydrogen is boxed in blue, which is more consistent with the coloring scheme seen on the right side of the periodic table. All of the elements found to the right of the metalloids are classified as non-metals, as is hydrogen. Hydrogen is unique, in that it shares some qualities with the elements on the right side of the periodic table, but its electronic properties align with those in Group 1A. Ultimately, hydrogen's placement on the periodic table was based on its electronic, rather than its physical, characteristics.

