11.2 Hydrocarbons
- Page ID
- 218438
Alkanes
We begin our study of organic chemistry with the hydrocarbons, the simplest organic compounds, which are composed of carbon and hydrogen atoms only. As we noted, there are several different kinds of hydrocarbons. They are distinguished by the types of bonding between carbon atoms and the properties that result from that bonding. Hydrocarbons with only carbon-to-carbon single bonds (C–C) and existing as a continuous chain of carbon atoms also bonded to hydrogen atoms are called alkanes (or saturated hydrocarbons). Saturated, in this case, means that each carbon atom is bonded to four other atoms (hydrogen or carbon)—the most possible; there are no double or triple bonds in the molecules.
The word saturated has the same meaning for hydrocarbons as it does for the dietary fats and oils: the molecule has no carbon-to-carbon double bonds (C=C).
We previously introduced the three simplest alkanes—methane (CH4), ethane (C2H6), and propane (C3H8) and they are shown again in Figure \(\PageIndex{1}\).
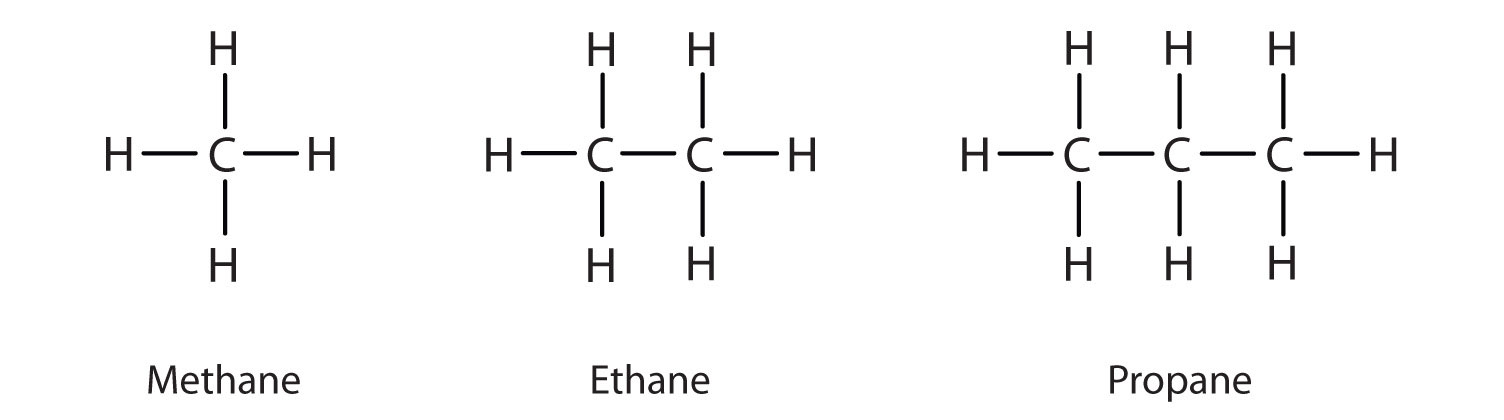
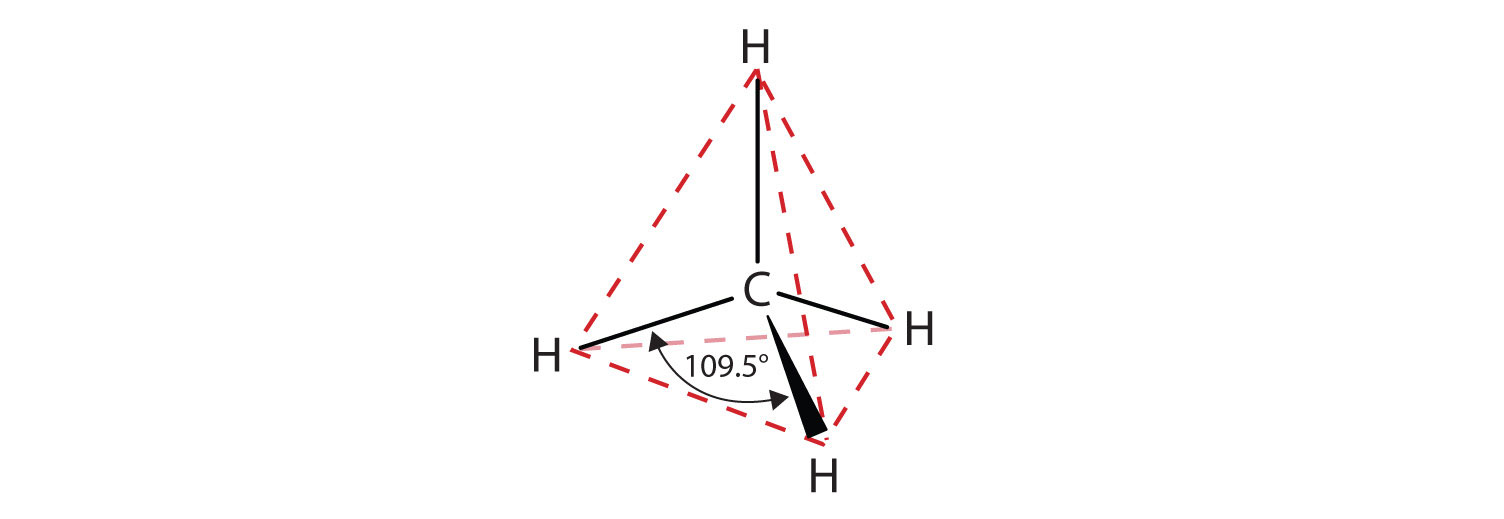
The flat representations shown do not accurately portray bond angles or molecular geometry. Methane has a tetrahedral shape that chemists often portray with wedges indicating bonds coming out toward you and dashed lines indicating bonds that go back away from you. An ordinary solid line indicates a bond in the plane of the page. Recall that the VSEPR theory correctly predicts a tetrahedral shape for the methane molecule (Figure \(\PageIndex{2}\)).
Methane (CH4), ethane (C2H6), and propane (C3H8) are the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH2 unit. The first 10 members of this series are given in Table \(\PageIndex{1}\).
| Name | Molecular Formula (CnH2n + 2) | Condensed Structural Formula | Number of Possible Isomers |
|---|---|---|---|
| methane | CH4 | CH4 | — |
| ethane | C2H6 | CH3CH3 | — |
| propane | C3H8 | CH3CH2CH3 | — |
| butane | C4H10 | CH3CH2CH2CH3 | 2 |
| pentane | C5H12 | CH3CH2CH2CH2CH3 | 3 |
| hexane | C6H14 | CH3CH2CH2CH2CH2CH3 | 5 |
| heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 | 9 |
| octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 | 18 |
| nonane | C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | 35 |
| decane | C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | 75 |
Consider the series in Figure \(\PageIndex{3}\). The sequence starts with C3H8, and a CH2 unit is added in each step moving up the series. Any family of compounds in which adjacent members differ from each other by a definite factor (here a CH2 group) is called a homologous series. The members of such a series, called homologs, have properties that vary in a regular and predictable manner. The principle of homology gives organization to organic chemistry in much the same way that the periodic table gives organization to inorganic chemistry. Instead of a bewildering array of individual carbon compounds, we can study a few members of a homologous series and from them deduce some of the properties of other compounds in the series.
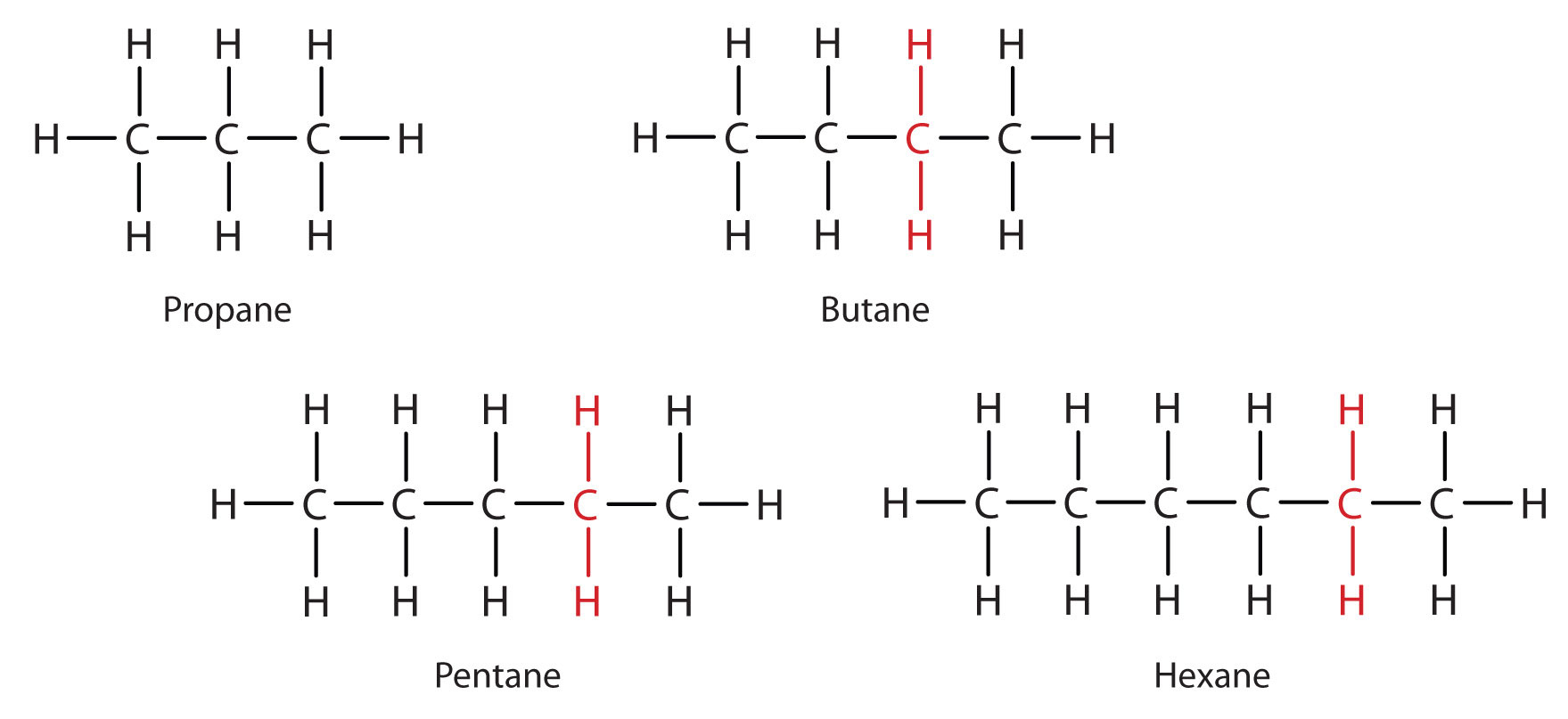
The principle of homology allows us to write a general formula for alkanes: CnH2n + 2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. For example, an alkane with eight carbon atoms has the molecular formula C8H(2 × 8) + 2 = C8H18.
Isomers
We can write the structure of butane (C4H10) by stringing four carbon atoms in a row,
–C–C–C–C–
and then adding enough hydrogen atoms to give each carbon atom four bonds:
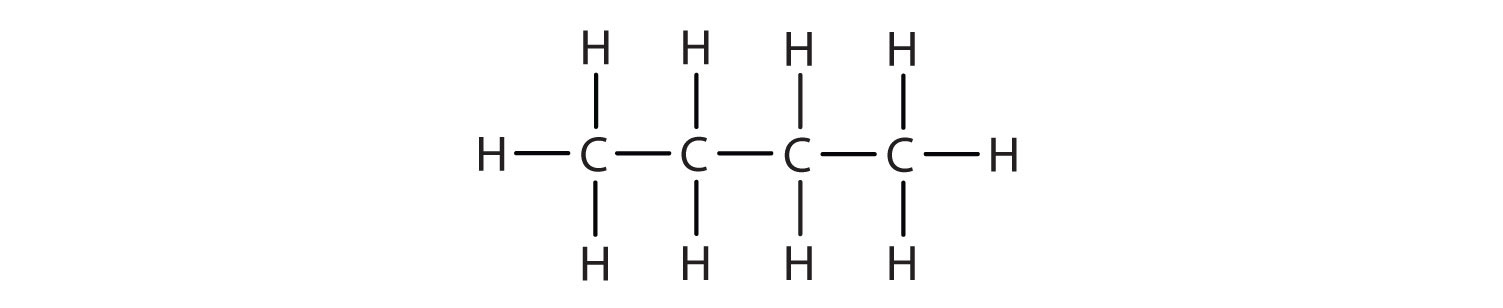
The compound butane has this structure, but there is another way to put 4 carbon atoms and 10 hydrogen atoms together. Place 3 of the carbon atoms in a row and then branch the fourth one off the middle carbon atom:
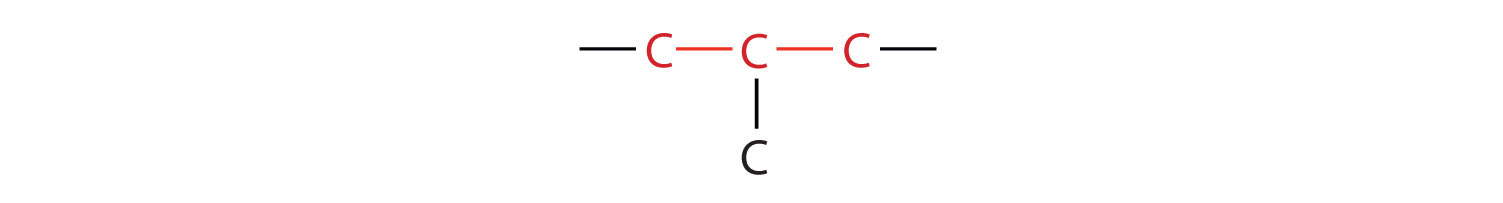
Now we add enough hydrogen atoms to give each carbon four bonds.
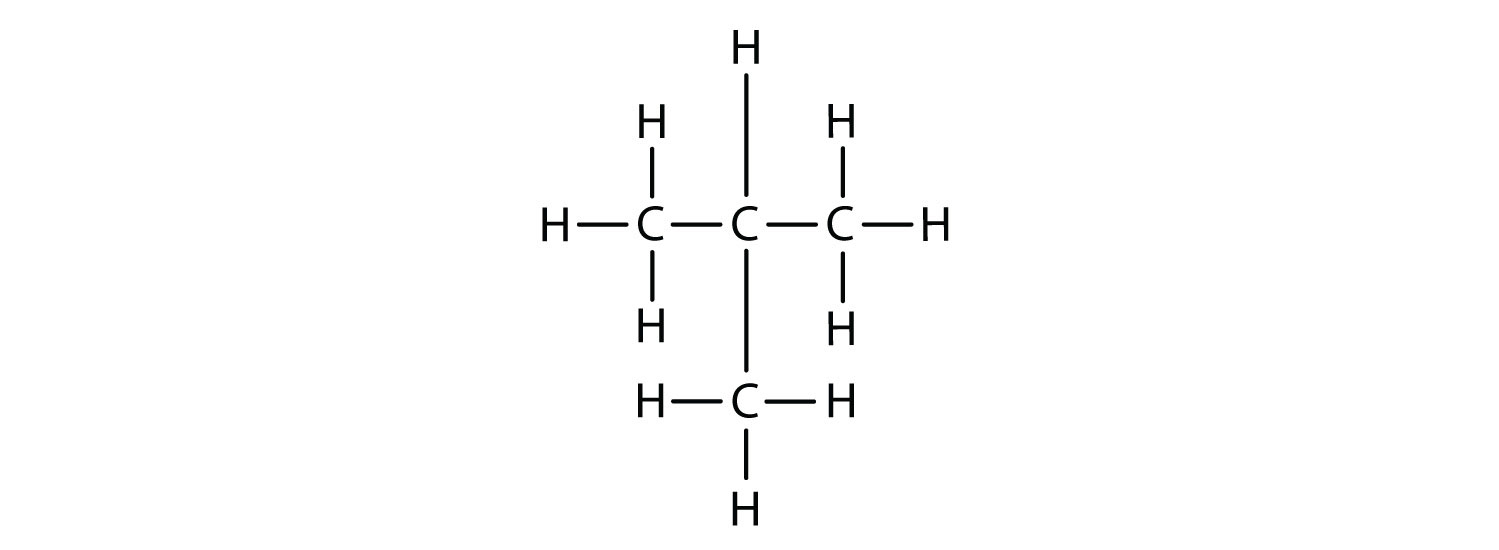
Notice that C4H10 is depicted with a bent chain in Figure \(\PageIndex{1}\). The four-carbon chain may be bent in various ways because the groups can rotate freely about the C–C bonds. However, this rotation does not change the identity of the compound. It is important to realize that bending a chain does not change the identity of the compound; all of the following represent the same compound, butane:
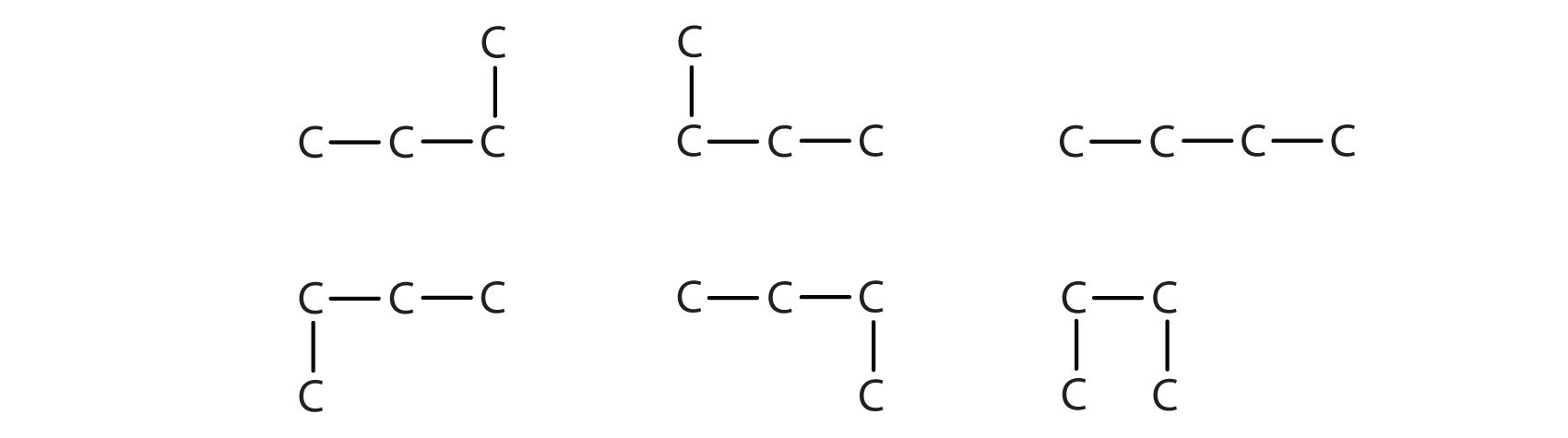
The structure of isobutane shows a continuous chain of three carbon atoms only, with the fourth attached as a branch off the middle carbon atom of the continuous chain, which is different from the structures of butane (compare the two structures in Figure \(\PageIndex{1}\).
Unlike C4H10, the compounds methane (CH4), ethane (C2H6), and propane (C3H8) do not exist in isomeric forms because there is only one way to arrange the atoms in each formula so that each carbon atom has four bonds.
Next beyond C4H10 in the homologous series is pentane. Each compound has the same molecular formula: C5H12. (Table 12.2 has a column identifying the number of possible isomers for the first 10 straight-chain alkanes.) The compound at the far left is pentane because it has all five carbon atoms in a continuous chain. The compound in the middle is isopentane; like isobutane, it has a one CH3 branch off the second carbon atom of the continuous chain. The compound at the far right, discovered after the other two, was named neopentane (from the Greek neos, meaning “new”). Although all three have the same molecular formula, they have different properties, including boiling points: pentane, 36.1°C; isopentane, 27.7°C; and neopentane, 9.5°C.
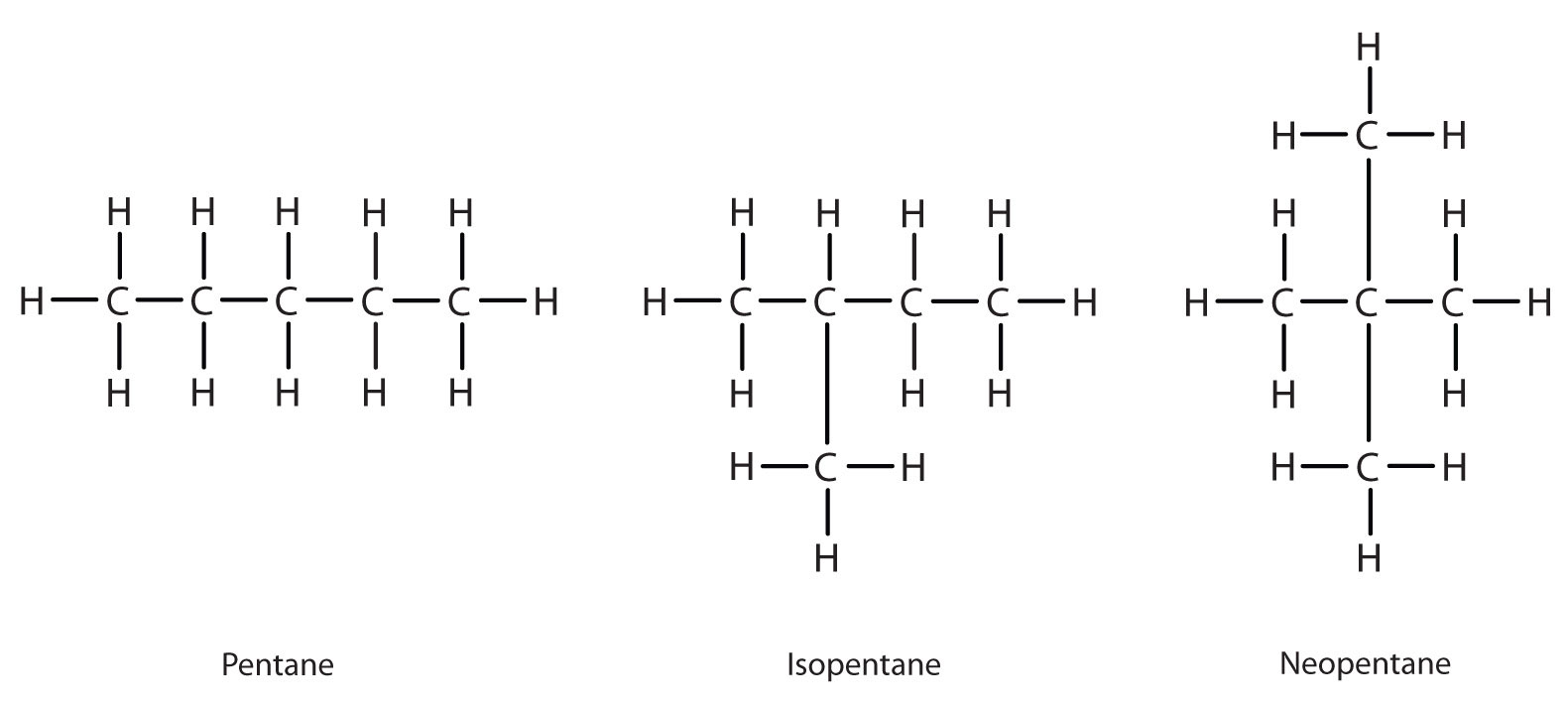
A continuous (unbranched) chain of carbon atoms is often called a straight chain even though the tetrahedral arrangement about each carbon gives it a zigzag shape. Straight-chain alkanes are sometimes called normal alkanes, and their names are given the prefix n-. For example, butane is called n-butane. We will not use that prefix here because it is not a part of the system established by the International Union of Pure and Applied Chemistry.
Condensed Structural and Line-AngleFormulas
We use several kinds of formulas to describe organic compounds. A molecular formula shows only the kinds and numbers of atoms in a molecule. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms.
Unfortunately, structural formulas are difficult to type/write and take up a lot of space. Chemists often use condensed structural formulas to alleviate these problems. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated for butane:

The ultimate condensed formula is a line-angle formula, in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. For example, we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as follows:

Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom.
Alkenes
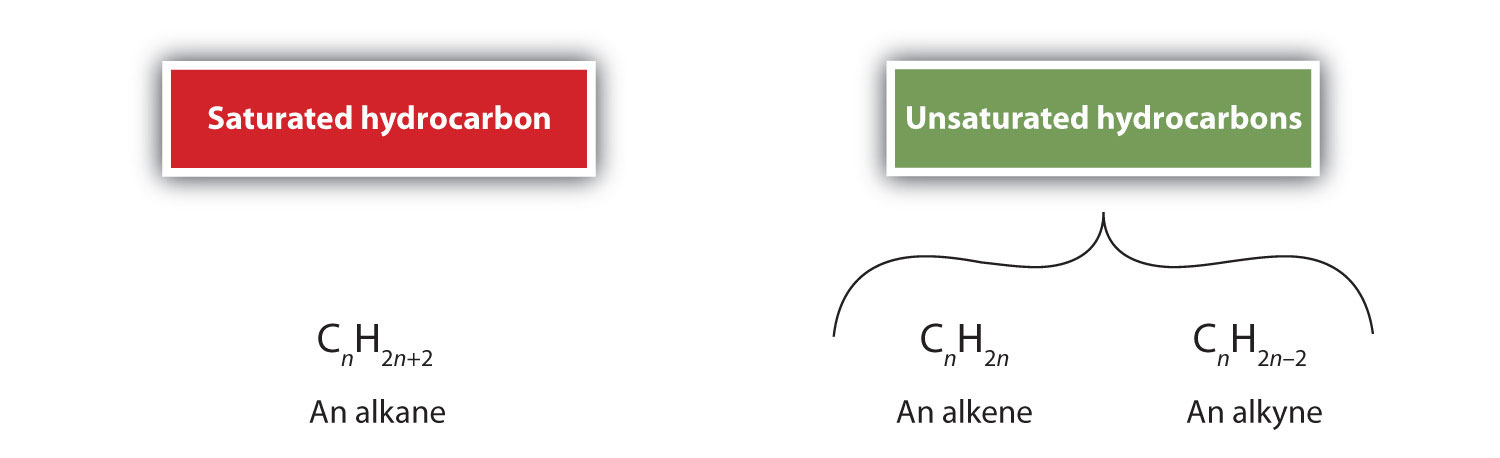
Alkenes are hydrocarbons with carbon-to-carbon double bonds (R2C=CR2) and alkynes are hydrocarbons with carbon-to-carbon triple bonds (R–C≡C–R). Collectively, they are called unsaturated hydrocarbons because they have fewer hydrogen atoms than does an alkane with the same number of carbon atoms, as is indicated in the following general formulas:
Some representative alkenes—their names, structures, and physical properties—are given in Table \(\PageIndex{1}\).
| IUPAC Name | Molecular Formula | Condensed Structural Formula | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|
| ethene | C2H4 | CH2=CH2 | –169 | –104 |
| propene | C3H6 | CH2=CHCH3 | –185 | –47 |
| 1-butene | C4H8 | CH2=CHCH2CH3 | –185 | –6 |
| 1-pentene | C5H10 | CH2=CH(CH2)2CH3 | –138 | 30 |
| 1-hexene | C6H12 | CH2=CH(CH2)3CH3 | –140 | 63 |
| 1-heptene | C7H14 | CH2=CH(CH2)4CH3 | –119 | 94 |
| 1-octene | C8H16 | CH2=CH(CH2)5CH3 | –102 | 121 |
We used only condensed structural formulas in Table \(\PageIndex{1}\). Thus, CH2=CH2 stands for
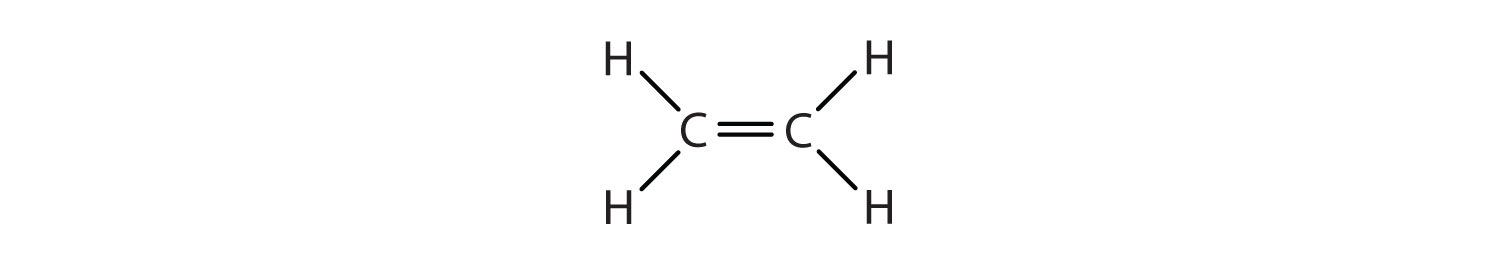
The double bond is shared by the two carbons and does not involve the hydrogen atoms, although the condensed formula does not make this point obvious. Note that the molecular formula for ethene is C2H4, whereas that for ethane is C2H6.
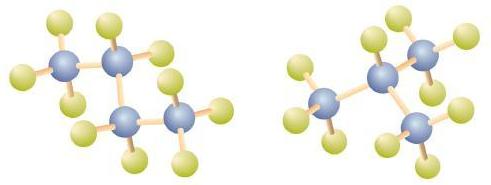
The first two alkenes in Table \(\PageIndex{1}\), ethene and propene, are most often called by their common names—ethylene and propylene, respectively (Figure \(\PageIndex{1}\)). Ethylene is a major commercial chemical. The US chemical industry produces about 25 billion kilograms of ethylene annually, more than any other synthetic organic chemical. More than half of this ethylene goes into the manufacture of polyethylene, one of the most familiar plastics. Propylene is also an important industrial chemical. It is converted to plastics, isopropyl alcohol, and a variety of other products.

Figure \(\PageIndex{1}\): Ethene and Propene. The ball-and-spring models of ethene/ethylene (a) and propene/propylene (b) show their respective shapes, especially bond angles.
Alkynes
The simplest alkyne—a hydrocarbon with carbon-to-carbon triple bond—has the molecular formula C2H2 and is known by its common name—acetylene (Figure \(\PageIndex{1}\)). Its structure is H–C≡C–H.
Acetylene is used in oxyacetylene torches for cutting and welding metals. The flame from such a torch can be very hot. Most acetylene, however, is converted to chemical intermediates that are used to make vinyl and acrylic plastics, fibers, resins, and a variety of other products.
Alkynes are similar to alkenes in both physical and chemical properties. For example, alkynes undergo many of the typical addition reactions of alkenes. The International Union of Pure and Applied Chemistry (IUPAC) names for alkynes parallel those of alkenes, except that the family ending is -yne rather than -ene. The IUPAC name for acetylene is ethyne.
Aromatics
Next we consider a class of hydrocarbons with molecular formulas like those of unsaturated hydrocarbons, but which, unlike the alkenes, do not readily undergo addition reactions. These compounds comprise a distinct class, called aromatic hydrocarbons, with unique structures and properties. We start with the simplest of these compounds. Benzene (C6H6) is of great commercial importance, but it also has noteworthy health effects.
The formula C6H6 seems to indicate that benzene has a high degree of unsaturation. (Hexane, the saturated hydrocarbon with six carbon atoms has the formula C6H14—eight more hydrogen atoms than benzene.) However, despite the seeming low level of saturation, benzene is rather unreactive. It does not, for example, react readily with bromine, which, is a test for unsaturation.
Benzene is a liquid that smells like gasoline, boils at 80°C, and freezes at 5.5°C. It is the aromatic hydrocarbon produced in the largest volume. It was formerly used to decaffeinate coffee and was a significant component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. It was removed from many product formulations in the 1950s, but others continued to use benzene in products until the 1970s when it was associated with leukemia deaths. Benzene is still important in industry as a precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent for such things as cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited.
To explain the surprising properties of benzene, chemists suppose the molecule has a cyclic, hexagonal, planar structure of six carbon atoms with one hydrogen atom bonded to each. We can write a structure with alternate single and double bonds, either as a full structural formula or as a line-angle formula:
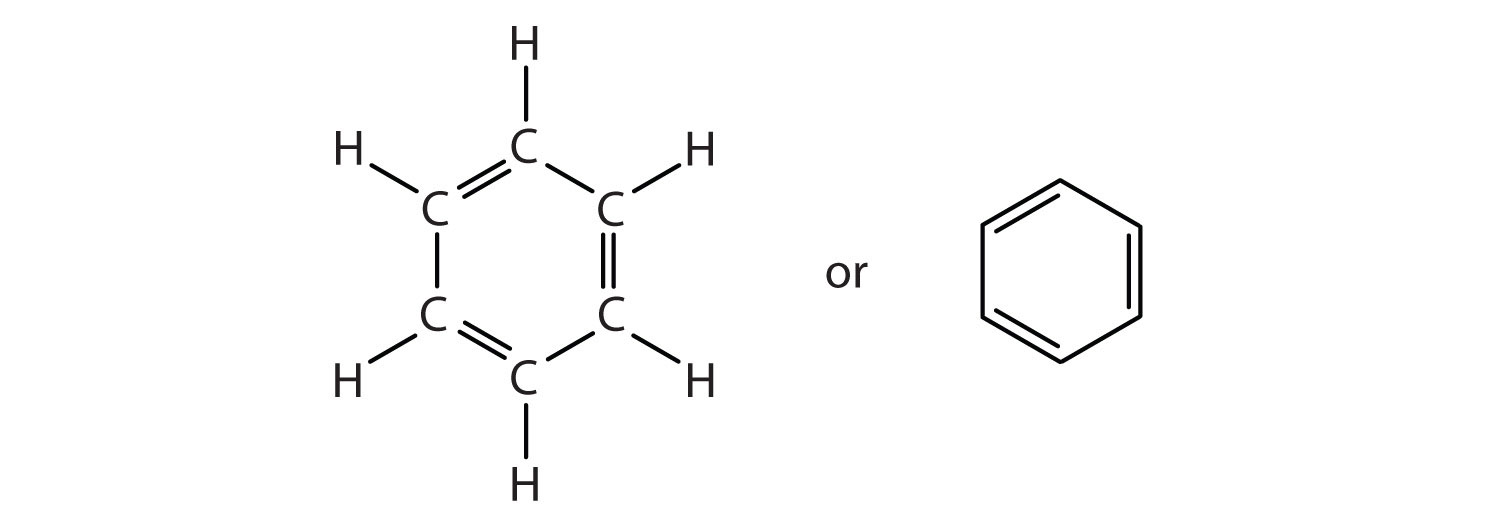
However, these structures do not explain the unique properties of benzene. Furthermore, experimental evidence indicates that all the carbon-to-carbon bonds in benzene are equivalent, and the molecule is unusually stable. Chemists often represent benzene as a hexagon with an inscribed circle:
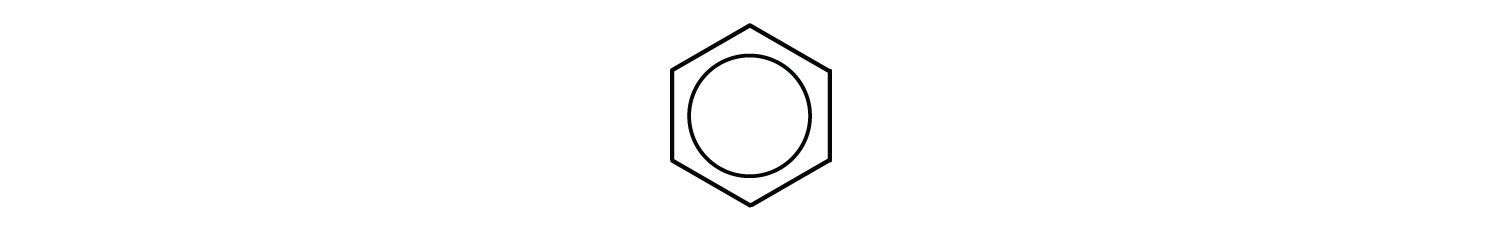
The inner circle indicates that the valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized, or spread out, over all the carbon atoms). It is understood that each corner of the hexagon is occupied by one carbon atom, and each carbon atom has one hydrogen atom attached to it. Any other atom or groups of atoms substituted for a hydrogen atom must be shown bonded to a particular corner of the hexagon. We use this modern symbolism, but many scientists still use the earlier structure with alternate double and single bonds.
To Your Health: Benzene and Us
Most of the benzene used commercially comes from petroleum. It is employed as a starting material for the production of detergents, drugs, dyes, insecticides, and plastics. Once widely used as an organic solvent, benzene is now known to have both short- and long-term toxic effects. The inhalation of large concentrations can cause nausea and even death due to respiratory or heart failure, while repeated exposure leads to a progressive disease in which the ability of the bone marrow to make new blood cells is eventually destroyed. This results in a condition called aplastic anemia, in which there is a decrease in the numbers of both the red and white blood cells.

