2.E: Unsaturated Hydrocarbons (Exercises)
- Page ID
- 338687
Additional Exercises
-
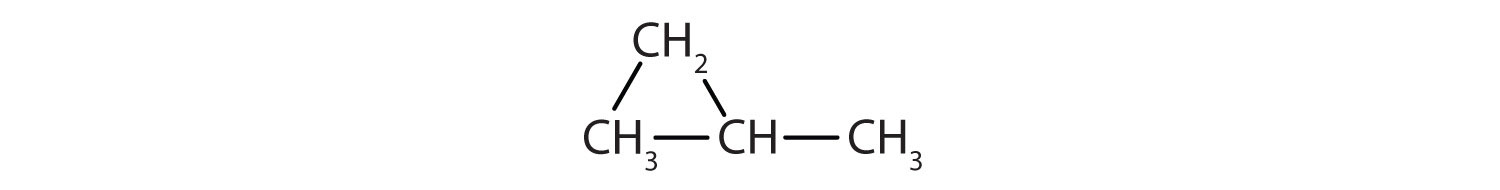
Classify each compound as saturated or unsaturated.
-
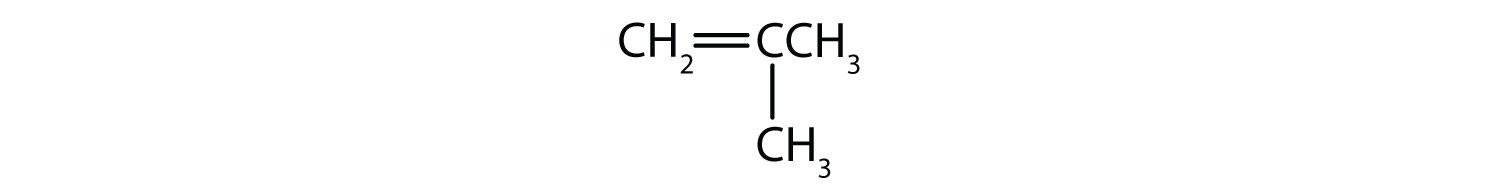
- CH3C≡CCH3
-
-
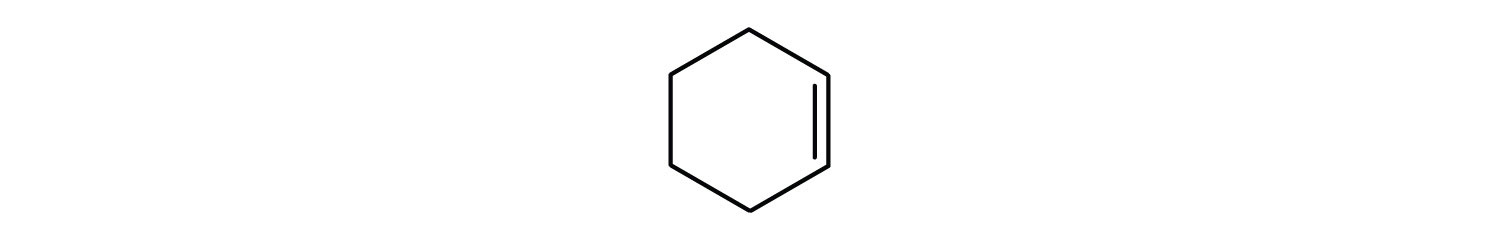
Classify each compound as saturated or unsaturated.
-
-
Give the molecular formula for each compound.
-
-
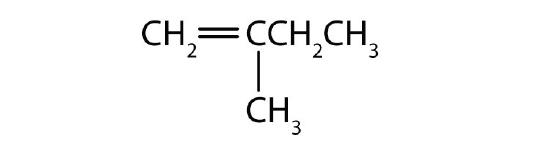
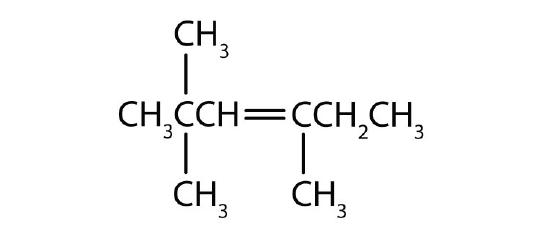
Name each compound according to the IUPAC system.
-
Draw and name all the alkene cis-trans isomers corresponding to the molecular formula C5H10. (Hint: there are only two.)
-
What is wrong with each name? Draw the structure and give the correct name for each compound.
- 2-methyl-4-heptene
- 2-ethyl-2-hexene
- 2,2-dimethyl-3-pentene
-
What is wrong with each name?
- 2-bromobenzene
- 3,3-dichlorotoluene
- 1,4-dimethylnitrobenzene
-
Following are line-angle formulas for three compounds. Draw the structure and give the name for each.
-
-
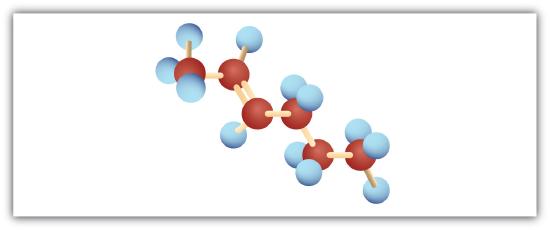
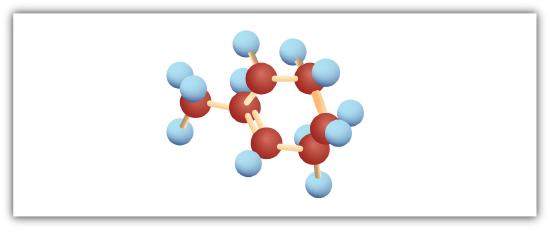
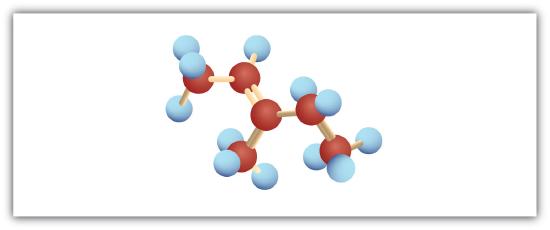
Following are ball-and-stick molecular models for three compounds (blue balls represent H atoms; red balls are C atoms). Provide the skeletal structure and give the name for each.
-
Answers
-
- unsaturated
- unsaturated
-
- C6H10
- C4H8
-
-
number not needed
-
can’t have two groups on one carbon atom on a benzene ring
-
can’t have a substituent on the same carbon atom as the nitro group
-
-
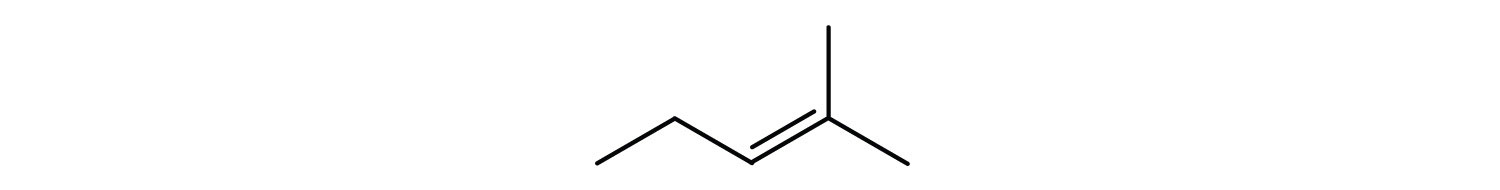
 ; 2-hexene
; 2-hexene
-
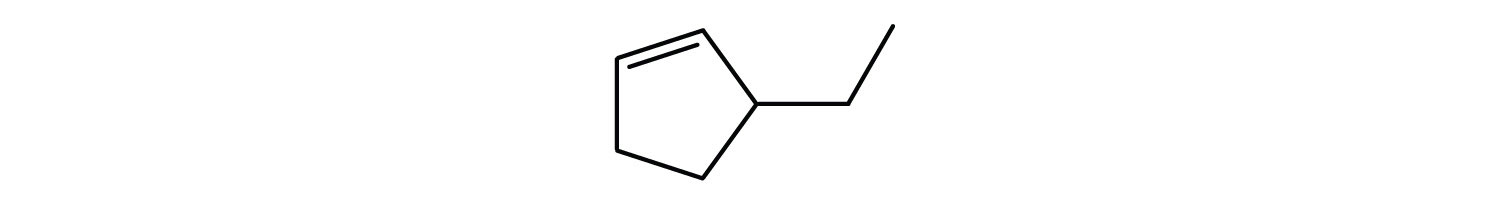
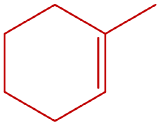 ; 1-methylcyclohexene
; 1-methylcyclohexene
-
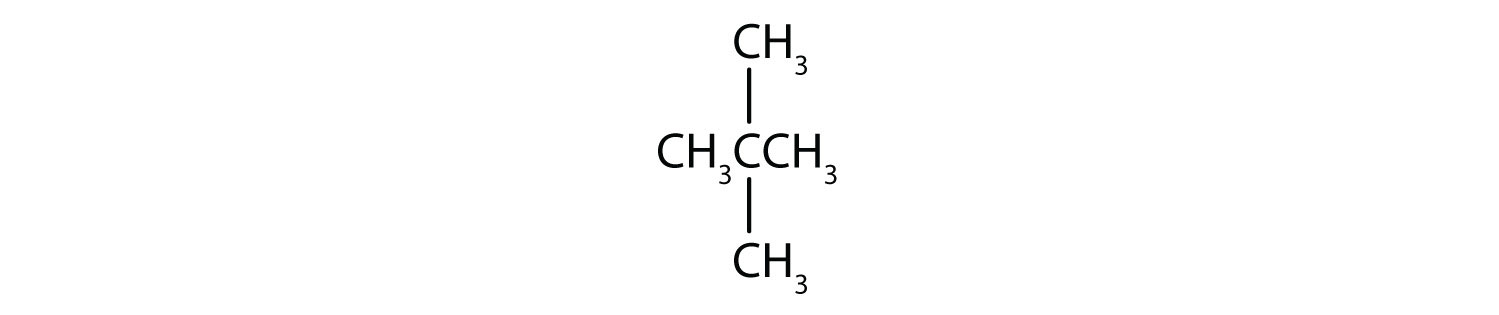
 ; 3-methyl-2-pentene
; 3-methyl-2-pentene


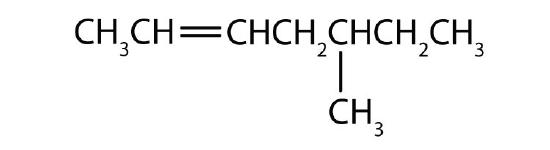
.png?revision=1&size=bestfit&width=411&height=76)