8.7: Enzyme Inhibition
- Page ID
- 342729
Learning Outcomes
- Define allosteric site.
- Distinguish between reversible and irreversible inhibitors.
- Distinguish between competitive and noncompetitive inhibitors.
- Define feedback inhibition.
In addition to concentration, pH, and, temperature; the presence of inhibitors will also affect enzyme activity. Inhibitors are compounds that cause enzymes to lose activity, either by slowing or stopping the chemical reaction. Some inhibitors cause temporary loss of activity, while others cause permanent loss of activity.
Reversible Inhibitors
A reversible inhibitor is one that will cause a temporary loss of enzymatic activity. This substance forms a non-covalent interaction with the enzyme. Reversible inhibitors can be competitive of noncompetitive.
A competitive inhibitor is any compound that bears a structural resemblance to a particular substrate and thus competes with that substrate for binding at the active site of an enzyme. The inhibitor is not acted on by the enzyme but does prevent the substrate from approaching the active site.
The degree to which a competitive inhibitor interferes with an enzyme’s activity depends on the relative concentrations of the substrate and the inhibitor. If the inhibitor is present in relatively large quantities, it will initially block most of the active sites. But because the binding is reversible, some substrate molecules will eventually bind to the active site and be converted to product. Increasing the substrate concentration promotes displacement of the inhibitor from the active site. Competitive inhibition can be completely reversed by adding substrate so that it reaches a much higher concentration than that of the inhibitor.
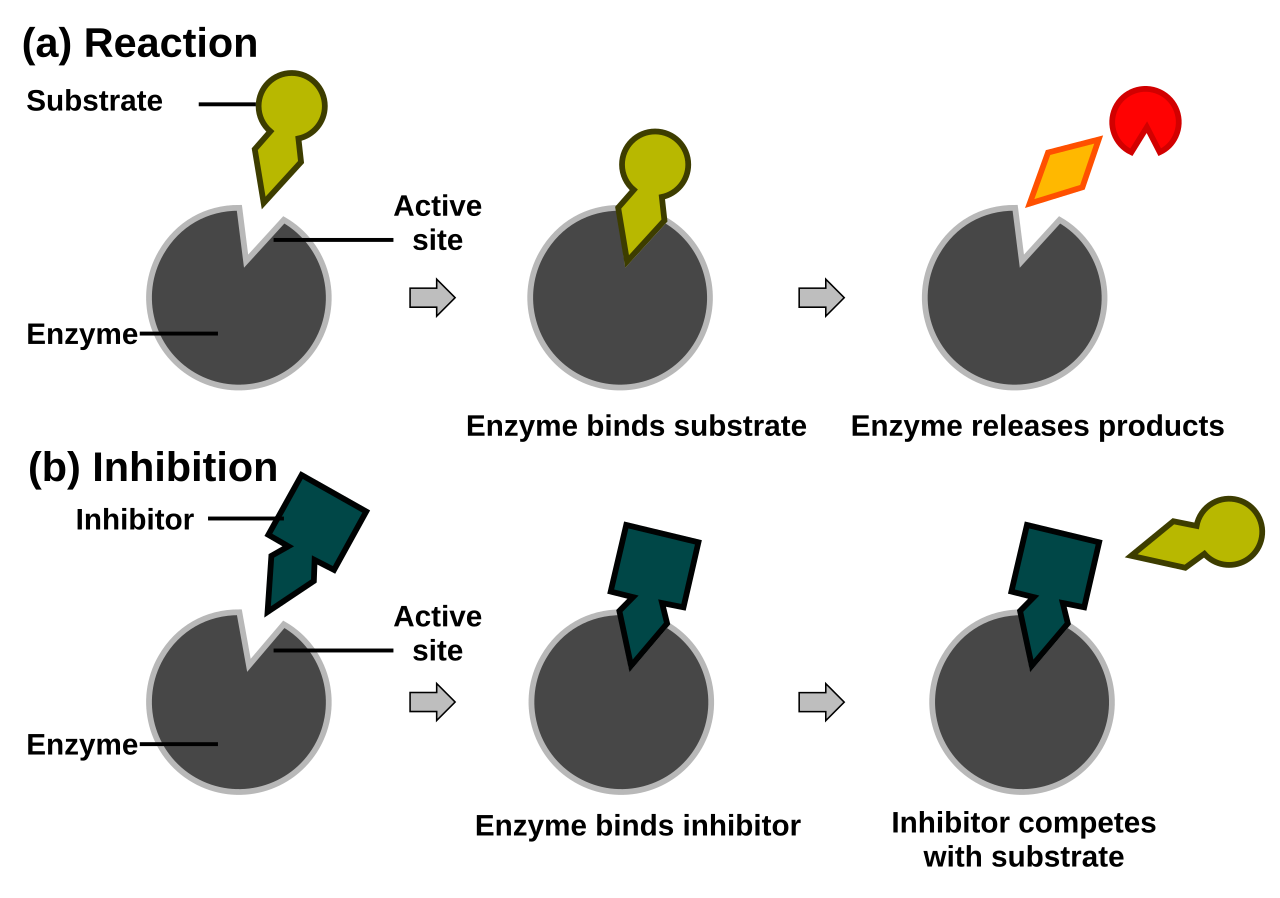
A noncompetitive inhibitor attaches at an allosteric site, which is any site on the enzyme that is not the active site. The attachment of the non-competitive inhibitor to the allosteric site results in a shift in three-dimensional structure that alters the shape of the active site so that the substrate will no longer fit in the active site properly (Figure \(\PageIndex{2}\)). A noncompetitive inhibitor can combine with either the free enzyme or the enzyme-substrate complex because its binding site on the enzyme is distinct from the active site. Because the inhibitor does not structurally resemble the substrate, the addition of excess substrate does not reverse the inhibitory effect.
Feedback inhibition is a normal biochemical process that makes use of noncompetitive inhibitors to control some enzymatic activity. In this process, the final product inhibits the enzyme that catalyzes the first step in a series of reactions. Feedback inhibition is used to regulate the synthesis of many amino acids. For example, bacteria synthesize isoleucine from threonine in a series of five enzyme-catalyzed steps. As the concentration of isoleucine increases, some of it binds as a noncompetitive inhibitor to the first enzyme of the series (threonine deaminase), thus bringing about a decrease in the amount of isoleucine being formed (Figure \(\PageIndex{3}\)).
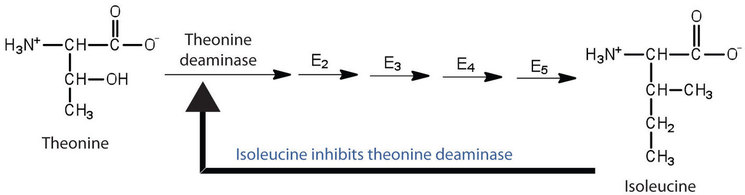
Figure \(\PageIndex{3}\): Feedback Inhibition of Threonine Deaminase by Isoleucine. Threonine deaminase is the first enzyme in the conversion of threonine to isoleucine. Isoleucine inhibits threonine deaminase through feedback inhibition.
Irreversible Inhibitors
An irreversible inhibitor is one that will cause a permanent loss of enzymatic activity. An irreversible inhibitor inactivates an enzyme by bonding covalently to a particular group at the active site. The inhibitor-enzyme bond is so strong that the inhibition cannot be reversed by the addition of excess substrate. The nerve gases, especially Diisopropyl fluorophosphate (DIFP), irreversibly inhibit biological systems by forming an enzyme-inhibitor complex with a specific OH group of serine situated at the active sites of certain enzymes. The peptidases trypsin and chymotrypsin contain serine groups at the active site and are inhibited by DIFP.
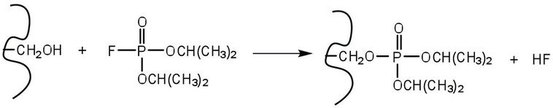
Figure \(\PageIndex{4}\): An irreversible inhibitor forming a covalent bond.
Example \(\PageIndex{1}\)
What are the characteristics of an irreversible inhibitor?
Solution
It inactivates an enzyme by bonding covalently to a particular group at the active site.
Exercise \(\PageIndex{1}\)
In what ways does a competitive inhibitor differ from a noncompetitive inhibitor?
Summary
An irreversible inhibitor inactivates an enzyme by bonding covalently to a particular group at the active site. A reversible inhibitor inactivates an enzyme through noncovalent, reversible interactions. A competitive inhibitor competes with the substrate for binding at the active site of the enzyme. A noncompetitive inhibitor binds at a site distinct from the active site.
Supplemental Resources
- Enzymes: https://youtu.be/E90D4BmaVJM
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)

