8.2: EPA's Primary and Secondary Drinking Water Standards
- Page ID
- 85171
- Recognize the accomplishments of the Safe Water Drinking Act (original law and amendments)
- Distinguish between primary and secondary contaminants.
- Classify a contaminant as being organic, inorganic, disinfectant, disinfectant byproduct, radionuclide, or microbial.
- Distinguish between Maximum Contaminant Level (MCL) and Maximum Contaminant Level Goal (MCLG) drinking water standards.
- Recognize contaminant units- ppm, ppb, picoCuries, MFL, and millirems.
- Recall who monitors tap water, well water, and bottled water.
The Safe Water Drinking Act (1974)
Up until 1974, public drinking water supplies in the United States were monitored and regulated by state and local authorities. Lists of contaminants with their various concentrations could vary from state to state. As the chemical industry grew, these same state agencies noted the increased presence of existing and new organic chemicals in public water systems. In order to standardize drinking water across the country, the Environmental Protection Agency (EPA) enacted the Safe Water Drinking Act of 1974.

The 1974 act enabled the EPA to monitor and regulate public water systems that serve over 25 people. Implementation and enforcement of drinking water standards would still be performed by each state. Regarding drinking water sources (surface and ground), the EPA and state agencies protect and monitor these as well. Levels of contaminants would be defined using the concentration terms Maximum Contaminant Level (MCL) and Treatment Technique (TT)
The first set of drinking water standards included only 22 chemicals and/or pathogens. EPA established two major types of contaminants: primary and secondary. The first of these types (primary) contaminants are substances (examples could include Hg, As, and U) that can be toxic in small amounts. On the other hand, secondary contaminants are less toxic species (Fe and Zn) and would include cosmetic issues (color, taste, and odor) of drinking water.
All primary contaminants have enforceable concentration values. For the majority of these pollutants, EPA lists concentration limits by using the term Maximum Contaminant Level (MCL). If a water supplier exceeds a given MCL for a toxin, then fines and penalties could by imposed by the EPA. A few pathogens (Giardia Lamblia and Legionella) use Treatment Technique (TT) notation rather than numerical MCL concentrations. Water that contains any amount of these pathogens must be sanitized immediately with a standardized EPA procedure.
| Primary Contaminant | Classification |
MCLG1(mg/L) |
MCL or TT1(mg/L) | Potential Health Effects from Long-Term Exposure Above the MCL (unless specified as short-term) | Sources of Contaminant in Drinking Water |
|---|---|---|---|---|---|
| Giardia lamblia | Microorganism | zero | TT3 | Gastrointestinal illness (such as diarrhea, vomiting, and cramps) | Human and animal fecal waste |
| Thallium | Inorganic | 0.0005 | 0.002 | Hair loss; changes in blood; kidney, intestine, or liver problems | Leaching from ore-processing sites; discharge from electronics, glass, and drug factories |
In Table \(\PageIndex{1}\), a Maximum Contaminant Level Goal (MCLG) column is displayed for each of the two drinking water impurities. MCLG values are not enforced by the EPA. Instead, these concentrations are suggested values that water suppliers should strive to meet. Every six years, the EPA reviews each primary contaminant with its MCL standard. During these times, they analyze data in regards to health risk assessment. If they choose to lower a MCL (smaller value) of a contaminant, then less health issues should occur. Unfortunately, reducing a concentration requires more technology which will cost the supplier and the consumer more money. By providing MCLG limits, the EPA encourages a water company to gradually work towards lowering a toxin's concentration.
Primary Drinking Water Contaminants
The National Primary Drinking Water Regulations (NPDWR) are legally enforceable primary standards and treatment techniques that apply to public water systems. Primary standards and treatment techniques protect public health by limiting the levels of contaminants in drinking water. A partial list of of standards for different water contaminants is given in the tables below. A more comprehensive and updated list can be found on this link.
These types of toxins are classified into one of the six EPA classifications: microorganisms, disinfectants, disinfectant byproducts, inorganics, organics, or radionuclides. Primary contaminants are regulated because the have the capacity to do great harm to humans, plants, and animals. If a water distributor (must serve at least 200 homes) exceeds one of these mandated standards, then the EPA may impose a fine on the company.

Concentration Units for Pollutants/Toxins
Typically, concentrations of primary contaminants are listed in parts per million. Metric amounts of a part per million are milligrams of the toxin per liter of the water sample.
\[\text{ppm} = \dfrac{mg }{Liters } \nonumber \]
For extremely toxic substance, smaller units like parts per billion (ppb) might be used to express concentration. Metric amounts of a part per billion are micrograms of toxin per liter of water sample.
\[\text{ppb} = \dfrac{μg }{Liters } \nonumber \]
The EPA measures a few pollutants using different concentration units. For example, asbestos based products are reported in fibers per liter (MFL). This silica material has been used in the construction and in the automotive industries. The insulating and noncombustible nature of asbestos enabled builders to make homes that were less flammable. In addition, mechanics can install asbestos lined brakes that would not become flammable. Unfortunately, the wide spread use of asbestos has lead to lung diseases like mesothelioma and asbestos. Today, the EPA has placed limitations of asbestos, but has not banned the use of this material in some industries. For more current information of EPA's stance on asbestos limitations, please click on this link.

Other primary contaminants that utilize alternative concentration units are radio nuclides and disinfectant byproducts. Radio nuclides are materials that naturally emit radioactive particles or waves. Examples of these would include Ra-226 and Uranium. Both of these elements have unstable nuclei that produce ionizing radiation. Exposure to even tiny amounts of radio nuclides can increase a person's likelihood of developing cancer. For this reason, the EPA regulates ionizing radiation using small concentrations (ppb). In addition, the EPA uses units like millirem and picocuries for some radio nuclides. The unit millirem focuses on biological exposure to radiation. For information regarding the millirem and or Curie units, click on this OER link. As for picocurie, this term defines an isotope's rate of nuclei decomposition.
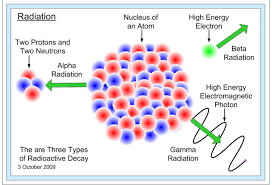
Chemicals used to disinfect drinking water and rid it of pathogens are regulated as primary contaminants. These types of compounds will react with the water to form trace amounts of carcinogen organohalides. For this reason, the EPA regulated disinfectants using Maximum Residual Disinfectant Level Goal (MRDLG) and Maximum Residual Disinfectant Level (MRDL) rather than MCLG and MCL. Once again, the goal portion of this term is a future limit and the level is a fineable standard.
Toxicity of primary contaminants can be understood by looking at the MCL value. The lower the concentration, the more dangerous a contaminant is. Using the primary drinking water standards, determine which organic compound is the most toxic.
Solution
Dioxin is the most toxic organic compound listed on the primary drinking water standards..
Amendment SDWA of 1986
This change to the Safe Drinking Water Act established MCLGs and increased the total of regulated contaminants to 83. The EPA installed more monitoring devices to detect organic contaminants. Research was done to detect pathogens more effectively to reduce disease. Lastly, public notifications from water systems to the consumers would be made announced if severe water issues occurred.
Amendment of SDWA of 1996
This particular legislation provided more protection and assessment of water sources (lakes, river, streams). In addition, water companies were required to provide consumers with a water quality report. States could seek federal money for upgrading their water quality processes. Cost benefit analysis would be done to determine risk and reward of lowering the concentration of a contaminant. For small regulated water companies, more financial and technical assistance would be offered to help them maintain drinking water standards.
Non-regulated Water
The EPA does not regulate private wells which serve less than 25 people or bottled water in the United States. Well water monitoring is the responsibility of the well owner. As for bottled water, the Food and Drug Administration (FDA) oversees this commodity by using EPA drinking water standards.
Notes (taken from 14.6-Delmar Link)
1Definitions:
- Maximum Contaminant Level Goal (MCLG) - The level of a contaminant in drinking water below which there is no known or expected risk to health. MCLGs allow for a margin of safety and are non-enforceable public health goals.
- Maximum Contaminant Level (MCL) - The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. MCLs are enforceable standards.
- Maximum Residual Disinfectant Level Goal (MRDLG) - The level of a drinking water disinfectant below which there is no known or expected risk to health. MRDLGs do not reflect the benefits of the use of disinfectants to control microbial contaminants.
- Treatment Technique (TT) - A required process intended to reduce the level of a contaminant in drinking water.
- Maximum Residual Disinfectant Level (MRDL) - The highest level of a disinfectant allowed in drinking water. There is convincing evidence that addition of a disinfectant is necessary for control of microbial contaminants.
2 Units are in milligrams per liter (mg/L) unless otherwise noted. Milligrams per liter are equivalent to parts per million (PPM).


