8.9: Fluoridation of Drinking Water
- Page ID
- 87262
- Recognize the formula of the fluoride ion.
- Name advantages of adding fluoride to munipal drinking water supplies.
- Understand how fluoride affects tooth enamel.
- Name disadvantages of water exhibiting higher levels of fluoride.
- Calculate and compare a sample's fluoride concentration to the EPA's MCL primary drinking water standard.
The mineral fluoride occurs naturally on earth and is released from rocks into the soil, water, and air. All water contains some fluoride. Usually, the fluoride level in water is not enough to prevent tooth decay; however, some groundwater and natural springs can have naturally high levels of fluoride. The picture below illustrate Fluorite crystals which incorporate the fluoride ion (F-).
.jpg?revision=1&size=bestfit&width=328&height=426)
History of Fluoride in Water
In the 1930's, scientists examined the relationship between tooth decay in children and naturally occurring fluoride in drinking water. This study found that children who drank water with naturally high levels of fluoride had less tooth decay. This discovery was important because during that time most children and adults in the United States were affected by tooth decay. Many suffered from toothaches and painful extractions—often losing permanent teeth, including molars, even as teenagers.
After much scientific research, in 1945, the city of Grand Rapids, Michigan, was the first to add fluoride to its city water system in order to provide residents with the benefits of fluoride. Since 1945, hundreds of cities have started community water fluoridation By 2012 (more than 210 million people), nearly 75% of the United States served by community water systems had access to fluoridated water. Because of its contribution to the dramatic decline in tooth decay over the past 70 years, CDC named community water fluoridation as 1 of 10 great public health achievements of the 20th century.
Figure \(\PageIndex{2}\): Fluoride in dental health. Image taken from: https://media.defense.gov/2018/Mar/19/2001891929/-1/-1/0/180323-F-PO640-008.JPG
How does Fluoride Work?
Fluoride protects teeth from decay. Bacteria in the mouth produce acid when a person eats sugary foods. This acid eats away minerals from the tooth’s surface, making the tooth weaker and increasing the chance of developing cavities. Fluoride helps to rebuild and strengthen the tooth’s surface, or enamel. Water fluoridation prevents tooth decay by providing frequent and consistent contact with low levels of fluoride. By keeping the tooth strong and solid, fluoride stops cavities from forming and can even rebuild the tooth’s surface.
.png?revision=1&size=bestfit&width=684&height=968)
Although other fluoride-containing products, such as toothpaste, mouth rinses, and dietary supplements are available and contribute to the prevention and control of tooth decay, community water fluoridation has been identified as the most cost-effective method of delivering fluoride to all, reducing tooth decay by 25% in children and adults.
EPA Monitoring of F-
It costs around 50 cents a year per person to fluoridate water for those who live in large communities. This additive is classified as a primary inorganic drinking water contaminant. The EPA has set both Maximum Contaminant Level (MCL) and Maximum Contaminant Level Goal (MCLG) concentrations for fluoride to be 4 ppm.
Your apartment complex's water contains 25 µg of F- per 10 mL of water. Does this value exceed the EPA MCL value for F-?
- Answer
-
The MCL EPA standard is 4 ppm. Recall that the units for ppm are mg/L. This problem provides a contaminant concentration in micrograms per liter. You will need to calculate the ppm concentration for the water sample and then compare this to 4 ppm. For the math below, I chose to calculate concentration in ppb and then convert it to ppm.
\[\begin{align*}10\,\cancel{ml} \times \dfrac{1\,L}{10^{3}\,\cancel{ml}} &= .01\,L \\[5pt] &= 1.0\times 10^{-2}\,L \end{align*} \]
\[\text{2500 ppb}= \dfrac{\text{25 µg Solute}}{\text{.01 L Solution}} \nonumber \]
We can easily obtain ppm by dividing ppb by 1000. Our final ppm concentration for the fluoride sample is 2.5 ppm. This does not exceed the MCL standard.
Negative effects of F-
There is some debate as to lowering this concentrations. Fluorosis, overexposure to fluoride, can cause pitting and staining of the teeth. This condition is more prevalent in younger individuals. In addition, excess fluoride can affect bone strength. For these reasons, the EPA monitors the amount of fluoride found in water to achieve optimal prevention of tooth decay.
.png)
Figure \(\PageIndex{4}\): Mild Fluorosis in a younger dental patient. Image taken from: en.Wikipedia.org/wiki/Dental_fluorosis
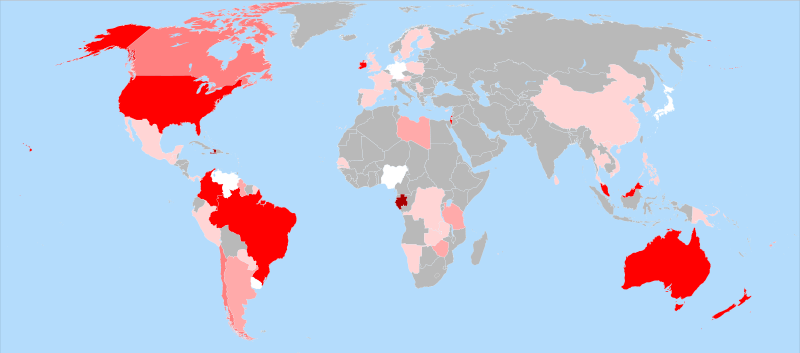
Fluoride in the World
Many countries naturally have fluoride deposits in their water. For others, fluoride is added to drinking water to reduces cavities. Looking at the map below, the areas that are shaded in darker red pigments have fluoride (either naturally or artificially) in the tap water (brown red: 80-100% population, bright red: 60-80% population, lighter red: 20-40% population, pink: 1-20% population, white: less than 1%, and grey: unknown.
References
1)https://uwaterloo.ca/scholar/cchieh/home
2) Andy Brunning Compound Interest



