3.E: Covalent Bonding and Simple Molecular Compounds (Exercises)
- Page ID
- 218310
Additional Exercises
Use the atomic masses found in Figure 2.7.1
- An atomic mass unit equals 1.661 × 10−24 g. What is the mass in grams of each molecule of (a) H2S (b) N2O4 (c) ICl3 (d) NCl3?
- An atomic mass unit equals 1.661 × 10−24 g. What is the mass in grams of (a) O2F2 (b) CCl4 (c) C6H6 (d) SO3?
- An atomic mass unit equals 1.661 × 10−24 g. What is the mass in grams of 5.00 × 1022 molecules of C9H8O4?
- An atomic mass unit equals 1.661 × 10−24 g. What is the mass in grams of 1.885 × 1020 molecules of C27H46O?
- Acetic acid has the following structure:
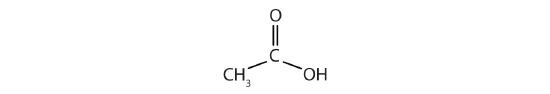
This molecule can lose a hydrogen ion (H+) and the resulting anion can combine with other cations, such as Na+:
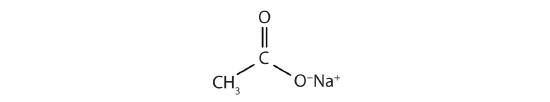
Name this ionic compound.
- Formic acid (HCOOH) loses a hydrogen ion to make the formate ion (HCOO−). Write the formula for each ionic compound: potassium formate, calcium formate, and ferric formate.
- Cyanogen has the formula C2N2. Propose a bonding scheme that gives each atom the correct number of covalent bonds. (Hint: the two carbon atoms are in the center of a linear molecule.)
- How many carbon–carbon single bonds, linked together, are needed to make a carbon chain that is 1.000 cm long?
- How many carbon–carbon double bonds, linked together, are needed to make a carbon chain that is 1.000 cm long?
- In addition to themselves, what other atoms can carbon atoms bond with and make covalent bonds that are nonpolar (or as nonpolar as possible)?
- What is the greatest possible electronegativity difference between any two atoms? Use Figure 4.4 to find the answer.
- Acetaminophen, a popular painkiller, has the following structure:
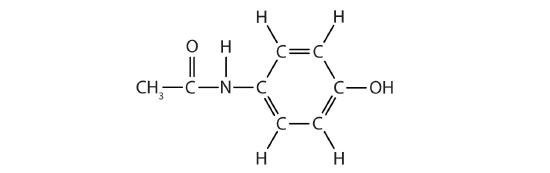
Name the recognizable functional groups in this molecule. Do you think there are other groups of atoms in this molecule that might qualify as functional groups?
- Glutamic acid is the parent compound of monosodium glutamate (known as MSG), which is used as a flavor enhancer. Glutamic acid has the following structure:
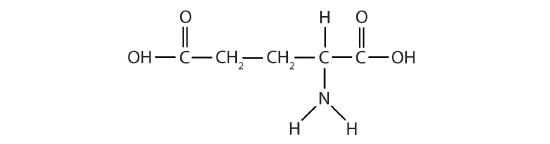
Name the functional groups you recognize in this molecule. Do you think there are other groups of atoms in this molecule that might qualify as functional groups?
Answers
1.
a: 5.661 × 10−23 g
b: 1.534 × 10−22 g
c: 3.874 × 10−22 g
d: 1.999 × 10−22 g
2.
a: 1.163 × 10−22 g
b: 2.555 × 10−22 g
c: 1.298 × 10−22 g
d: 1.330 × 10−22 g
3. 14.96 g
4. 0.1211 g
5. sodium acetate
6.
a. KHCOO
b. Ca(HCOO)2
c. Fe(HCOO)3
- :N≡C–C≡N:
- 6.49 × 107 C-C bonds
- 7.46 × 107 C=C bonds
- Hydrogen atoms make relatively nonpolar bonds with carbon atoms.
- The greatest electronegativity difference is 3.2, between F and Rb.
- alcohol; the ring with double bonds, and the O=C-NH are also likely functional groups.
- carboxyl and -NH2 functional groups

