3.1.1.0: Atomic Orbitals and Quantum Numbers (Problems)
- Page ID
- 210687
PROBLEM \(\PageIndex{1}\)
What are the allowed values for each of the four quantum numbers: n, l, ml, and ms?
- Answer
-
n: non-zero integer
l: 0 to n-1
ml : -l to l
ms : \(\dfrac{1}{2}\) or \(\dfrac{-1}{2}\)
PROBLEM \(\PageIndex{2}\)
Describe the properties of an electron associated with each of the following four quantum numbers: n, l, ml, and ms.
- Answer
-
n determines the general range for the value of energy and the probable distances that the electron can be from the nucleus. l determines the shape of the orbital. ml determines the orientation of the orbitals of the same l value with respect to one another. ms determines the spin of an electron
PROBLEM \(\PageIndex{3}\)
Identify the subshell in which electrons with the following quantum numbers are found:
- n = 2, l = 1
- n = 4, l = 2
- n = 6, l = 0
- Answer a
-
2p
- Answer b
-
4d
- Answer c
-
6s
PROBLEM \(\PageIndex{4}\)
Identify the subshell in which electrons with the following quantum numbers are found:
- n = 3, l = 2
- n = 1, l = 0
- n = 4, l = 3
- Answer a
-
3d
- Answer b
-
1s
- Answer c
-
4f
PROBLEM \(\PageIndex{5}\)
Consider the orbitals shown here in outline.
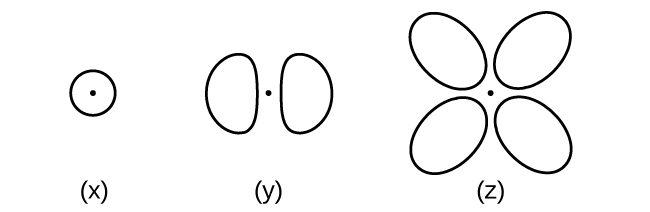
- What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
- How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)?
- Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell with n = 2. Of an orbital of type (z) in a shell with n = 3.
- What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)?
- What are the possible l and ml values for an orbital of type (x)? Of type (y)? Of type (z)?
- Answer a
-
x. 2
y. 2
z. 2
- Answer b
-
x. 1
y. 3
z. 0
- Answer c
-
x. 4 0 0 \(\dfrac{1}{2}\)
y. 2 1 0 \(\dfrac{1}{2}\)
z. 3 2 0 \(\dfrac{1}{2}\)
- Answer d
-
x. 1
y. 2
z. 3
- Answer e
-
x. l = 0, ml = 0
y. l = 1, ml = –1, 0, or +1
z. l = 2, ml = –2, –1, 0, +1, +2
PROBLEM \(\PageIndex{6}\)
How many electrons could be held in the second shell of an atom if the spin quantum number ms could have three values instead of just two? (Hint: Consider the Pauli exclusion principle.)
- Answer
-
12
PROBLEM \(\PageIndex{7}\)
Write a set of quantum numbers for each of the electrons with an n of 4 in a Se atom.
- Answer
-
n l ml s 4 0 0 \(+\dfrac{1}{2}\) 4 0 0 \(−\dfrac{1}{2}\) 4 1 −1 \(+\dfrac{1}{2}\) 4 1 0 \(+\dfrac{1}{2}\) 4 1 +1 \(+\dfrac{1}{2}\) 4 1 −1 \(−\dfrac{1}{2}\)
PROBLEM \(\PageIndex{8}\)
Answer the following questions:
- Without using quantum numbers, describe the differences between the shells, subshells, and orbitals of an atom.
- How do the quantum numbers of the shells, subshells, and orbitals of an atom differ?
- Answer a
-
shell: set of orbitals in the same energy level
subshell: set of orbitals in the same energy level and same shape (s, p, d, or f)
orbital: can hold up to 2 electrons
- Answer b
-
shell: set of orbitals with same n
subshell: set of orbitals in an atom with the same values of n and l
orbital: shape defined by l quantum number
PROBLEM \(\PageIndex{9}\)
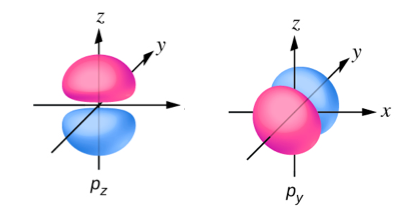
Sketch the boundary surface of a pz and a py orbital. Be sure to show and label the axes.
- Answer
-
PROBLEM \(\PageIndex{10}\)
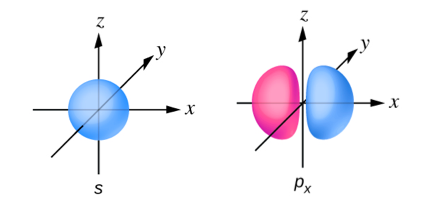
Sketch the px and s orbitals. Be sure to show and label the coordinates.
- Answer
-
Have a video solution request?
Let your professors know here.
***Please know that you are helping future students - videos will be made in time for next term's class.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
Feedback
Think one of the answers above is wrong? Let us know here.

