2.5.2: Solutions Chemistry
- Page ID
- 210653
Learning Objectives
- Describe the basic properties of solutions and how they form
- Predict whether a given mixture will yield a solution based on molecular properties of its components
- Explain why some solutions either produce or absorb heat when they form
- Define and give examples of electrolytes
The previous unit introduced solutions, defined as homogeneous mixtures of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution present in relatively lesser concentrations are called solutes. Sugar is a covalent solid composed of sucrose molecules, \(\mathrm{C_{12}H_{22}O_{11}}\). When this compound dissolves in water, its molecules become uniformly distributed among the molecules of water:
\[\mathrm{C_{12}H_{22}O}_{11(s)}⟶\mathrm{C_{12}H_{22}O}_{11(aq)} \label{Eq1}\]
The subscript “aq” in the equation signifies that the sucrose molecules are solutes and are therefore individually dispersed throughout the aqueous solution (water is the solvent). Although sucrose molecules are heavier than water molecules, they remain dispersed throughout the solution; gravity does not cause them to “settle out” over time.
Potassium dichromate, \(\mathrm{K_2Cr_2O_7}\), is an ionic compound composed of colorless potassium ions, \(\mathrm{K^+}\), and orange dichromate ions, \(\mathrm{Cr_2O_7^{2−}}\). When a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly distributed throughout the mixture (Figure \(\PageIndex{1}\)), as indicated in this equation:
\[\mathrm{K_2Cr_2O}_{7(s)}⟶\mathrm{2K^+}_{(aq)}+\mathrm{Cr_2O_7^{2-}}_{(aq)} \label{Eq2}\]
As with the mixture of sugar and water, this mixture is also an aqueous solution. Its solutes, potassium and dichromate ions, remain individually dispersed among the solvent (water) molecules.

Figure \(\PageIndex{1}\): When potassium dichromate (\(K_2Cr_2O_7\)) is mixed with water, it forms a homogeneous orange solution. (credit: modification of work by Mark Ott).
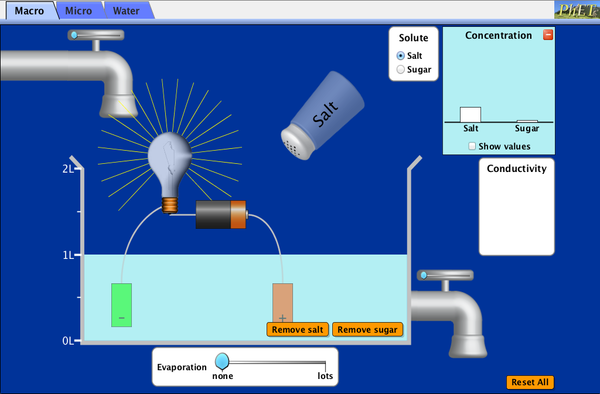
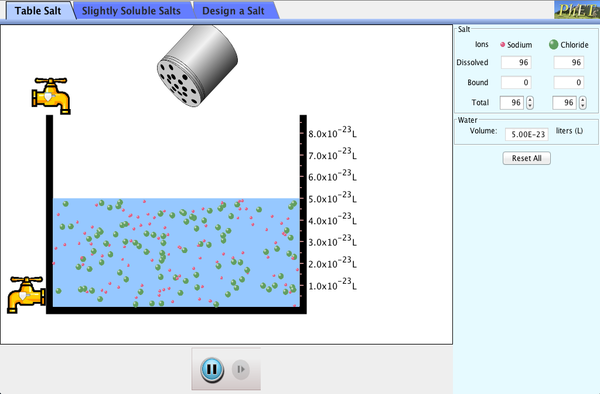
Learn More about The dissolution of covalent and ionic compounds
Click the link to use a simulator.
Water is used so often as a solvent that the word solution has come to imply an aqueous solution to many people. However, almost any gas, liquid, or solid can act as a solvent. Many alloys are solid solutions of one metal dissolved in another; for example, US five-cent coins contain nickel dissolved in copper. Air is a gaseous solution, a homogeneous mixture of nitrogen, oxygen, and several other gases. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Table \(\PageIndex{1}\) gives examples of several different solutions and the phases of the solutes and solvents.
| Solution | Solute | Solvent |
|---|---|---|
| air | O2(g) | N2(g) |
| soft drinks | CO2(g) | H2O(l) |
| hydrogen in palladium | H2(g) | Pd(s) |
| rubbing alcohol | H2O(l) | C3H8O(l) (2-propanol) |
| saltwater | NaCl(s) | H2O(l) |
| brass | Zn(s) | Cu(s) |
Solutions exhibit these defining traits:
- They are homogeneous; that is, after a solution is mixed, it has the same composition at all points throughout (its composition is uniform).
- The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in Table \(\PageIndex{1}\).
- The components of a solution are dispersed on a molecular scale; that is, they consist of a mixture of separated molecules, atoms, and/or ions.
- The dissolved solute in a solution will not settle out or separate from the solvent.
- The composition of a solution, or the concentrations of its components, can be varied continuously, within limits.
The Formation of Solutions
The formation of a solution is an example of a spontaneous process, a process that occurs under specified conditions without the requirement of energy from some external source. Sometimes we stir a mixture to speed up the dissolution process, but this is not necessary; a homogeneous solution would form if we waited long enough. The topic of spontaneity is critically important to the study of chemical thermodynamics and is treated more thoroughly in Unit 8. For purposes of this chapter’s discussion, it will suffice to consider two criteria that favor, but do not guarantee, the spontaneous formation of a solution:
- a decrease in the internal energy of the system (an exothermic change, as discussed in the previous chapter on thermochemistry)
- an increase in the disorder in the system (which indicates an increase in the entropy of the system, as you will learn about in the later chapter on thermodynamics)
In the process of dissolution, an internal energy change often, but not always, occurs as heat is absorbed or evolved. An increase in disorder always results when a solution forms.
When containers of helium and argon are connected, the gases spontaneously mix due to diffusion and form a solution (Figure \(\PageIndex{2}\)). The formation of this solution clearly involves an increase in disorder, since the helium and argon atoms occupy a volume twice as large as that which each occupied before mixing.
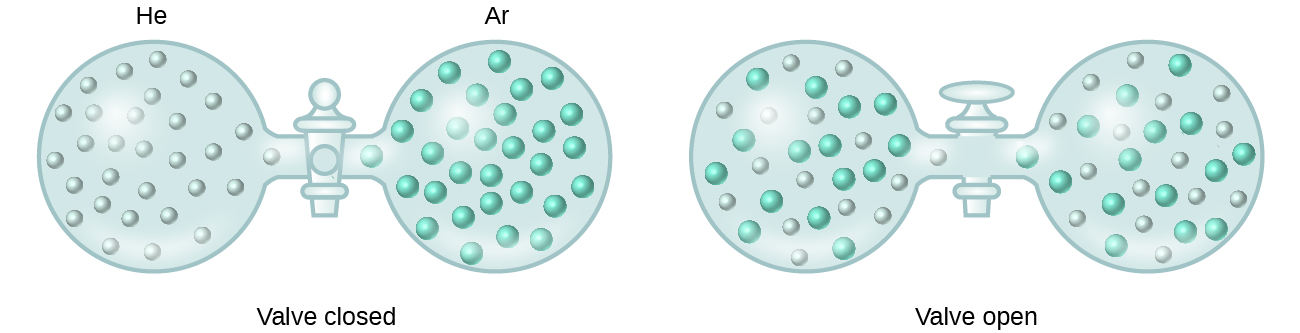
Figure \(\PageIndex{2}\): Samples of helium and argon spontaneously mix to give a solution in which the disorder of the atoms of the two gases is increased.
Ideal solutions may also form when structurally similar liquids are mixed. For example, mixtures of the alcohols methanol (CH3OH) and ethanol (C2H5OH) form ideal solutions, as do mixtures of the hydrocarbons pentane, C5H12, and hexane, C6H14. Placing methanol and ethanol, or pentane and hexane, in the bulbs shown in Figure \(\PageIndex{2}\) will result in the same diffusion and subsequent mixing of these liquids as is observed for the He and Ar gases (although at a much slower rate), yielding solutions with no significant change in energy. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution.

Figure \(\PageIndex{3}\): This schematic representation of dissolution shows a stepwise process involving the endothermic separation of solute and solvent species (Steps 1 and 2) and exothermic solvation (Step 3).
As illustrated in Figure \(\PageIndex{3}\), the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome endothermic processes we will discuss in Part II of this course and released in an exothermic process referred to as solvation. The relative magnitudes of the energy changes associated with these stepwise processes determine whether the dissolution process overall will release or absorb energy. In some cases, solutions do not form because the energy required to separate solute and solvent species is so much greater than the energy released by solvation.

Figure \(\PageIndex{4}\): A mixture of nonpolar cooking oil and polar water does not yield a solution. (credit: Gautam Dogra).
For example, cooking oils and water will not mix to any appreciable extent to yield solutions (Figure \(\PageIndex{4}\)). Since the polar water molecules and nonpolar oil molecules would not experience very strong attraction, very little energy would be released by solvation. On the other hand, a mixture of ethanol and water will mix in any proportions to yield a solution. In this case, the solvation process is sufficiently exothermic to compensate for the endothermic separations of solute and solvent molecules.
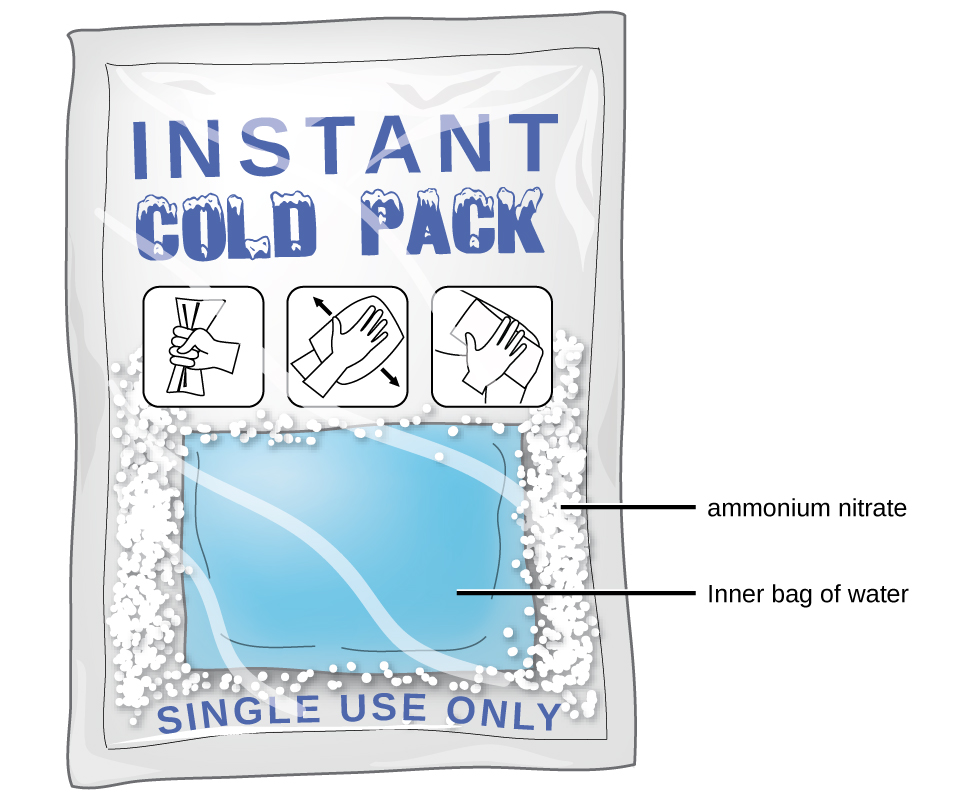
Figure \(\PageIndex{5}\): An instant cold pack gets cold when certain salts, such as ammonium nitrate, dissolve in water—an endothermic process.
As noted at the beginning of this module, spontaneous solution formation is favored, but not guaranteed, by exothermic dissolution processes. While many soluble compounds do, indeed, dissolve with the release of heat, some dissolve endothermically. Ammonium nitrate (NH4NO3) is one such example and is used to make instant cold packs for treating injuries like the one pictured in Figure \(\PageIndex{5}\). A thin-walled plastic bag of water is sealed inside a larger bag with solid NH4NO3. When the smaller bag is broken, a solution of NH4NO3 forms, absorbing heat from the surroundings (the injured area to which the pack is applied) and providing a cold compress that decreases swelling. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless due to the increase in disorder that accompanies formation of the solution.
Video \(\PageIndex{1}\): Watch this brief video illustrating endothermic and exothermic dissolution processes.
Electrolytes & Nonelectrolytes
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit (Figure \(\PageIndex{6}\)).
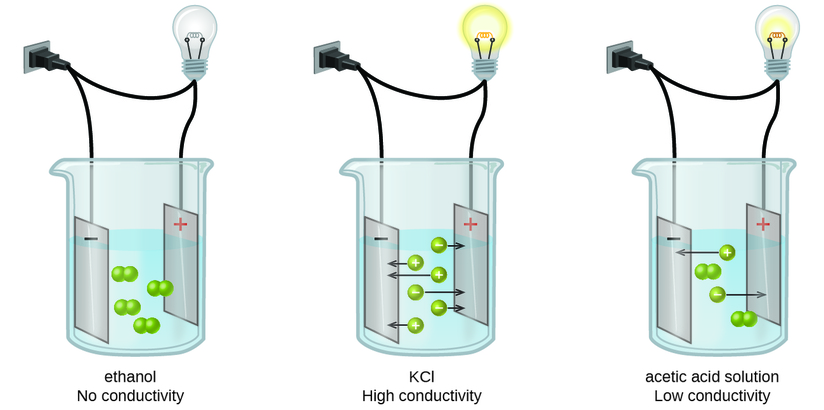
Figure \(\PageIndex{6}\): Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength of the electrolyte.
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure \(\PageIndex{7}\). We will further discuss these attractions in CHE 202.
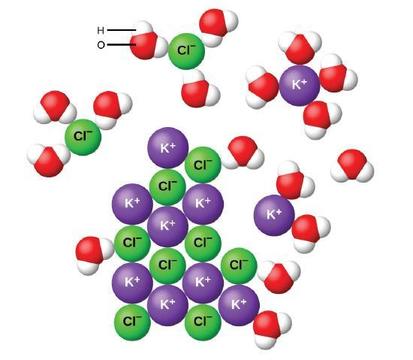
Figure \(\PageIndex{7}\): As potassium chloride (KCl) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the K+ and Cl− ions. Water molecules in front of and behind the ions are not shown.
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes.
Let us consider what happens at the microscopic level when we add solid KCl to water. Forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules penetrate between individual K+ and Cl− ions and surround them, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure shows. The reduction of the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution, resulting in an increase in the disorder of the system as the ions change from their fixed and ordered positions in the crystal to mobile and much more disordered states in solution. This increased disorder is responsible for the dissolution of many ionic compounds, including KCl, which dissolve with absorption of heat.
In other cases, the electrostatic attractions between the ions in a crystal are so large that the increase in disorder cannot compensate for the energy required to separate the ions, and the crystal is insoluble. Such is the case for compounds such as calcium carbonate (limestone), calcium phosphate (the inorganic component of bone), and iron oxide (rust).
Covalent Electrolytes
Pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25 °C. Water ionizes when one molecule of water gives up a proton to another molecule of water, yielding hydronium and hydroxide ions.
\[\ce{H_2O (l)+ H_2O (l) \rightleftharpoons H_3O^{+} (aq) + OH^{−} (aq)} \label{11.3.2}\]
In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl molecules. This gas contains no ions. However, when we dissolve hydrogen chloride in water, we find that the solution is a very good conductor. The water molecules play an essential part in forming ions: Solutions of hydrogen chloride in many other solvents, such as benzene, do not conduct electricity and do not contain ions.
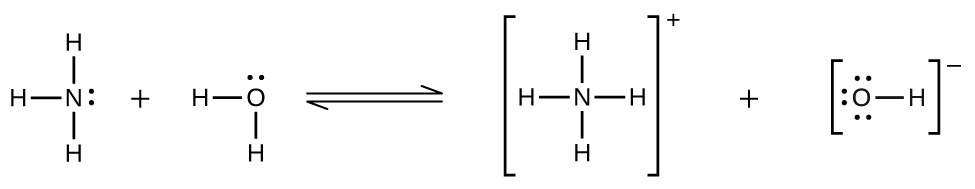
Hydrogen chloride is an acid, and so its molecules react with water, transferring H+ ions to form hydronium ions (\(H_3O^+\)) and chloride ions (Cl−):
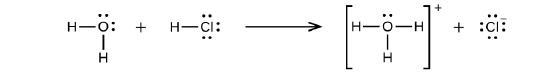
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.
Solubility
Imagine adding a small amount of salt to a glass of water, stirring until all the salt has dissolved, and then adding a bit more. You can repeat this process until the salt concentration of the solution reaches its natural limit. You can be certain that you have reached this limit because, no matter how long you stir the solution, undissolved salt remains. The concentration of salt in the solution at this point is known as its solubility.
The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium. Referring to the example of salt in water:
\[\ce{NaCl}(s)⇌\ce{Na+}(aq)+\ce{Cl-}(aq) \label{11.4.1}\]
When a solute’s concentration is equal to its solubility, the solution is said to be saturated with that solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A solution that contains a relatively low concentration of solute is called dilute, and one with a relatively high concentration is called concentrated.
If we add more salt to a saturated solution of salt, we see it fall to the bottom and no more seems to dissolve. In fact, the added salt does dissolve, as represented by the forward direction of the dissolution equation. Accompanying this process, dissolved salt will precipitate, as depicted by the reverse direction of the equation. The system is said to be at equilibrium when these two reciprocal processes are occurring at equal rates, and so the amount of undissolved and dissolved salt remains constant. Support for the simultaneous occurrence of the dissolution and precipitation processes is provided by noting that the number and sizes of the undissolved salt crystals will change over time, though their combined mass will remain the same.
Video \(\PageIndex{2}\): Watch this impressive video showing the precipitation of sodium acetate from a supersaturated solution.
Solutions may be prepared in which a solute concentration exceeds its solubility. Such solutions are said to be supersaturated, and they are interesting examples of nonequilibrium states. For example, the carbonated beverage in an open container that has not yet “gone flat” is supersaturated with carbon dioxide gas; given time, the CO2 concentration will decrease until it reaches its equilibrium value.
Solutions of Gases in Liquids
The chemical structures of the solute and solvent dictate the types of forces possible and, consequently, are important factors in determining solubility. For example, under similar conditions, the water solubility of oxygen is approximately three times greater than that of helium, but 100 times less than the solubility of chloromethane, CHCl3. Considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, C6H14, is approximately 20 times greater than it is in water.
Other factors also affect the solubility of a given substance in a given solvent. Temperature is one such factor, with gas solubility typically decreasing as temperature increases (Figure \(\PageIndex{8}\)). This is one of the major impacts resulting from the thermal pollution of natural bodies of water.
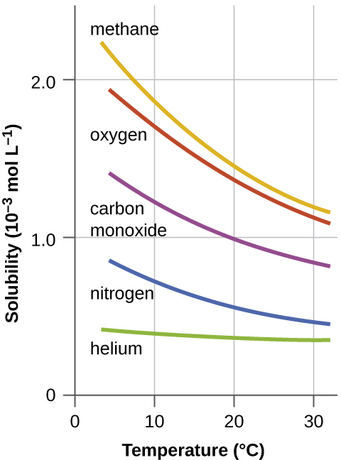
Figure \(\PageIndex{8}\): The solubilities of these gases in water decrease as the temperature increases. All solubilities were measured with a constant pressure of 101.3 kPa (1 atm) of gas above the solutions.
When the temperature of a river, lake, or stream is raised abnormally high, usually due to the discharge of hot water from some industrial process, the solubility of oxygen in the water is decreased. Decreased levels of dissolved oxygen may have serious consequences for the health of the water’s ecosystems and, in severe cases, can result in large-scale fish kills (Figure \(\PageIndex{9}\)).

Figure \(\PageIndex{9}\): (a) The small bubbles of air in this glass of chilled water formed when the water warmed to room temperature and the solubility of its dissolved air decreased. (b) The decreased solubility of oxygen in natural waters subjected to thermal pollution can result in large-scale fish kills. (credit a: modification of work by Liz West; credit b: modification of work by U.S. Fish and Wildlife Service)
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Gas solubility increases as the pressure of the gas increases. Carbonated beverages provide a nice illustration of this relationship. The carbonation process involves exposing the beverage to a relatively high pressure of carbon dioxide gas and then sealing the beverage container, thus saturating the beverage with CO2 at this pressure. When the beverage container is opened, a familiar hiss is heard as the carbon dioxide gas pressure is released, and some of the dissolved carbon dioxide is typically seen leaving solution in the form of small bubbles (Figure \(\PageIndex{10}\)). At this point, the beverage is supersaturated with carbon dioxide and, with time, the dissolved carbon dioxide concentration will decrease to its equilibrium value and the beverage will become “flat.”

Figure \(\PageIndex{10}\): Opening the bottle of carbonated beverage reduces the pressure of the gaseous carbon dioxide above the beverage. The solubility of CO2 is thus lowered, and some dissolved carbon dioxide may be seen leaving the solution as small gas bubbles. (credit: modification of work by Derrick Coetzee)
For many gaseous solutes, the relation between solubility, Cg, and partial pressure, Pg, is a proportional one:
where k is a proportionality constant that depends on the identities of the gaseous solute and solvent, and on the solution temperature. This is a mathematical statement of Henry’s law: The quantity of an ideal gas that dissolves in a definite volume of liquid is directly proportional to the pressure of the gas.
Case Study: Decompression Sickness (“The Bends”)
Decompression sickness (DCS), or “the bends,” is an effect of the increased pressure of the air inhaled by scuba divers when swimming underwater at considerable depths. In addition to the pressure exerted by the atmosphere, divers are subjected to additional pressure due to the water above them, experiencing an increase of approximately 1 atm for each 10 m of depth. Therefore, the air inhaled by a diver while submerged contains gases at the corresponding higher ambient pressure, and the concentrations of the gases dissolved in the diver’s blood are proportionally higher per Henry’s law.
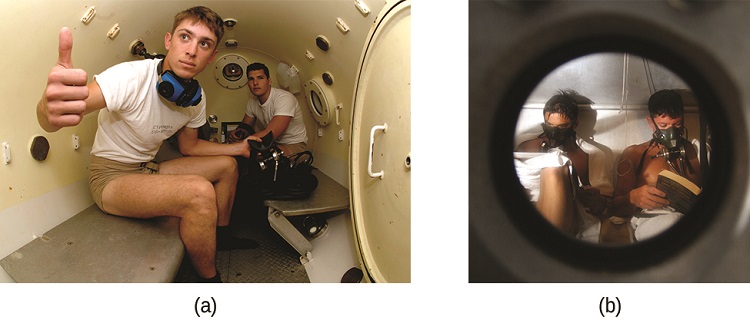
Figure \(\PageIndex{11}\): (a) US Navy divers undergo training in a recompression chamber. (b) Divers receive hyperbaric oxygen therapy.
As the diver ascends to the surface of the water, the ambient pressure decreases and the dissolved gases becomes less soluble. If the ascent is too rapid, the gases escaping from the diver’s blood may form bubbles that can cause a variety of symptoms ranging from rashes and joint pain to paralysis and death. To avoid DCS, divers must ascend from depths at relatively slow speeds (10 or 20 m/min) or otherwise make several decompression stops, pausing for several minutes at given depths during the ascent. When these preventive measures are unsuccessful, divers with DCS are often provided hyperbaric oxygen therapy in pressurized vessels called decompression (or recompression) chambers (Figure \(\PageIndex{11}\)).
Deviations from Henry’s law are observed when a chemical reaction takes place between the gaseous solute and the solvent. Thus, for example, the solubility of ammonia in water does not increase as rapidly with increasing pressure as predicted by the law because ammonia, being a base, reacts to some extent with water to form ammonium ions and hydroxide ions.
Gases can form supersaturated solutions. If a solution of a gas in a liquid is prepared either at low temperature or under pressure (or both), then as the solution warms or as the gas pressure is reduced, the solution may become supersaturated. In 1986, more than 1700 people in Cameroon were killed when a cloud of gas, almost certainly carbon dioxide, bubbled from Lake Nyos (Figure \(\PageIndex{12}\)), a deep lake in a volcanic crater. The water at the bottom of Lake Nyos is saturated with carbon dioxide by volcanic activity beneath the lake. It is believed that the lake underwent a turnover due to gradual heating from below the lake, and the warmer, less-dense water saturated with carbon dioxide reached the surface. Consequently, tremendous quantities of dissolved CO2 were released, and the colorless gas, which is denser than air, flowed down the valley below the lake and suffocated humans and animals living in the valley.

Figure \(\PageIndex{12}\): (a) It is believed that the 1986 disaster that killed more than 1700 people near Lake Nyos in Cameroon resulted when a large volume of carbon dioxide gas was released from the lake. (b) A CO2 vent has since been installed to help outgas the lake in a slow, controlled fashion and prevent a similar catastrophe from happening in the future. (credit a: modification of work by Jack Lockwood; credit b: modification of work by Bill Evans)
Solutions of Liquids in Liquids
We know that some liquids mix with each other in all proportions; in other words, they have infinite mutual solubility and are said to be miscible. Ethanol, sulfuric acid, and ethylene glycol (popular for use as antifreeze, pictured in Figure \(\PageIndex{13}\)) are examples of liquids that are completely miscible with water. Two-cycle motor oil is miscible with gasoline.

Figure \(\PageIndex{13}\): Water and antifreeze are miscible; mixtures of the two are homogeneous in all proportions. (credit: “dno1967”/Wikimedia commons)
Liquids that mix with water in all proportions are usually polar substances. Hence, the two kinds of molecules mix easily. Likewise, nonpolar liquids are miscible with each other. The solubility of polar molecules in polar solvents and of nonpolar molecules in nonpolar solvents is, again, an illustration of the chemical axiom “like dissolves like.”
On the Poison of a Puffer Fish
Video \(\PageIndex{3}\): Why puffer fish poison can be deadly.
Two liquids that do not mix to an appreciable extent are called immiscible. Layers are formed when we pour immiscible liquids into the same container. Gasoline, oil (Figure \(\PageIndex{14}\)), benzene, carbon tetrachloride, some paints, and many other nonpolar liquids are immiscible with water. The attraction between the molecules of such nonpolar liquids and polar water molecules is ineffectively weak. The only strong attractions in such a mixture are between the water molecules, so they effectively squeeze out the molecules of the nonpolar liquid. The distinction between immiscibility and miscibility is really one of degrees, so that miscible liquids are of infinite mutual solubility, while liquids said to be immiscible are of very low (though not zero) mutual solubility.

Figure \(\PageIndex{14}\): Water and oil are immiscible. Mixtures of these two substances will form two separate layers with the less dense oil floating on top of the water. (credit: “Yortw”/Flickr)
Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Two partially miscible liquids usually form two layers when mixed. In the case of the bromine and water mixture, the upper layer is water, saturated with bromine, and the lower layer is bromine saturated with water. Since bromine is nonpolar, and, thus, not very soluble in water, the water layer is only slightly discolored by the bright orange bromine dissolved in it. Since the solubility of water in bromine is very low, there is no noticeable effect on the dark color of the bromine layer (Figure \(\PageIndex{15}\)).

Figure \(\PageIndex{15}\): Bromine (the deep orange liquid on the left) and water (the clear liquid in the middle) are partially miscible. The top layer in the mixture on the right is a saturated solution of bromine in water; the bottom layer is a saturated solution of water in bromine. (credit: Paul Flowers)
Solutions of Solids in Liquids
The dependence of solubility on temperature for a number of inorganic solids in water is shown by the solubility curves in Figure \(\PageIndex{16}\). Reviewing these data indicate a general trend of increasing solubility with temperature, although there are exceptions, as illustrated by the ionic compound cerium sulfate.
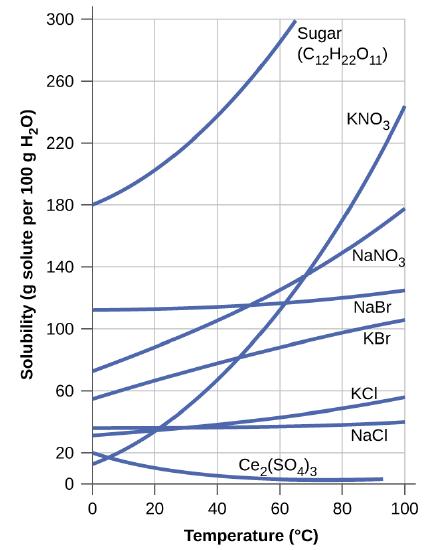
Figure \(\PageIndex{16}\): This graph shows how the solubility of several solids changes with temperature.
The temperature dependence of solubility can be exploited to prepare supersaturated solutions of certain compounds. A solution may be saturated with the compound at an elevated temperature (where the solute is more soluble) and subsequently cooled to a lower temperature without precipitating the solute. The resultant solution contains solute at a concentration greater than its equilibrium solubility at the lower temperature (i.e., it is supersaturated) and is relatively stable. Precipitation of the excess solute can be initiated by adding a seed crystal (see the video in the Link to Learning earlier in this module) or by mechanically agitating the solution. Some hand warmers, such as the one pictured in Figure \(\PageIndex{17}\), take advantage of this behavior.

Figure \(\PageIndex{17}\): This hand warmer produces heat when the sodium acetate in a supersaturated solution precipitates. Precipitation of the solute is initiated by a mechanical shockwave generated when the flexible metal disk within the solution is “clicked.” (credit: modification of work by “Velela”/Wikimedia Commons)
Video \(\PageIndex{4}\): This video shows the crystallization process occurring in a hand warmer.
Summary
Video \(\PageIndex{5}\): An overview of solutions and electrolytes.
A solution forms when two or more substances combine physically to yield a mixture that is homogeneous at the molecular level. The solvent is the most concentrated component and determines the physical state of the solution. The solutes are the other components typically present at concentrations less than that of the solvent. Solutions may form endothermically or exothermically, depending upon the relative magnitudes attractive forces we will discuss in the second term of this course. Ideal solutions form with no appreciable change in energy.
Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to only a small extent and are weak electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in water.
Video \(\PageIndex{6}\): An overview of solubility.
The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of intermolecular attractive forces that may exist between the substances’ atoms, ions, or molecules. This tendency to dissolve is quantified as substance’s solubility, its maximum concentration in a solution at equilibrium under specified conditions. A saturated solution contains solute at a concentration equal to its solubility. A supersaturated solution is one in which a solute’s concentration exceeds its solubility—a nonequilibrium (unstable) condition that will result in solute precipitation when the solution is appropriately perturbed. Miscible liquids are soluble in all proportions, and immiscible liquids exhibit very low mutual solubility. Solubilities for gaseous solutes decrease with increasing temperature, while those for most, but not all, solid solutes increase with temperature. The concentration of a gaseous solute in a solution is proportional to the partial pressure of the gas to which the solution is exposed, a relation known as Henry’s law.
Footnotes
- 1 If bubbles of gas are observed within the liquid, the mixture is not homogeneous and, thus, not a solution.
Key Equations
- \(C_\ce{g}=kP_\ce{g}\)
Glossary
- alloy
- solid mixture of a metallic element and one or more additional elements
- dissociation
- physical process accompanying the dissolution of an ionic compound in which the compound’s constituent ions are solvated and dispersed throughout the solution
- electrolyte
- substance that produces ions when dissolved in water
- Henry’s law
- law stating the proportional relationship between the concentration of dissolved gas in a solution and the partial pressure of the gas in contact with the solution
- ideal solution
- solution that forms with no accompanying energy change
- immiscible
- of negligible mutual solubility; typically refers to liquid substances
- miscible
- mutually soluble in all proportions; typically refers to liquid substances
- nonelectrolyte
- substance that does not produce ions when dissolved in water
- partially miscible
- of moderate mutual solubility; typically refers to liquid substances
- saturated
- of concentration equal to solubility; containing the maximum concentration of solute possible for a given temperature and pressure
- solubility
- extent to which a solute may be dissolved in water, or any solvent
- solvation
- exothermic process in which intermolecular attractive forces between the solute and solvent in a solution are established
- spontaneous process
- physical or chemical change that occurs without the addition of energy from an external source
- strong electrolyte
- substance that dissociates or ionizes completely when dissolved in water
- supersaturated
- of concentration that exceeds solubility; a nonequilibrium state
- unsaturated
- of concentration less than solubility
- weak electrolyte
- substance that ionizes only partially when dissolved in water
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
- Crash Course Chemistry: Crash Course is a division of Complexly and videos are free to stream for educational purposes.
- TED-Ed’s commitment to creating lessons worth sharing is an extension of TED’s mission of spreading great ideas. Within TED-Ed’s growing library of TED-Ed animations, you will find carefully curated educational videos, many of which represent collaborations between talented educators and animators nominated through the TED-Ed website.