1.5.3: Acid-Base Neutralization
- Page ID
- 210607
Learning Objectives
- Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral.
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. The word “neutralization” seems to imply that a stoichiometrically equivalent solution of an acid and a base would be neutral. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see.
Acid-Base Neutralization
A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. However, even if we mix stoichiometrically equivalent quantities, we may find that the resulting solution is not neutral. It could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed determines whether the solution is acidic, neutral, or basic. The following four situations illustrate how solutions with various pH values can arise following a neutralization reaction using stoichiometrically equivalent quantities:
- A strong acid and a strong base, such as HCl(aq) and NaOH(aq) will react to form a neutral solution since the conjugate partners produced are of negligible strength: \[\ce{HCl}(aq)+\ce{NaOH}(aq)⇌\ce{NaCl}(aq)+\ce{H2O}(l)\]
- A strong acid and a weak base yield a weakly acidic solution, not because of the strong acid involved, but because of the conjugate acid of the weak base.
- A weak acid and a strong base yield a weakly basic solution. A solution of a weak acid reacts with a solution of a strong base to form the conjugate base of the weak acid and the conjugate acid of the strong base. The conjugate acid of the strong base is a weaker acid than water and has no effect on the acidity of the resulting solution. However, the conjugate base of the weak acid is a weak base and ionizes slightly in water. This increases the amount of hydroxide ion in the solution produced in the reaction and renders it slightly basic.
- A weak acid plus a weak base can yield either an acidic, basic, or neutral solution. This is the most complex of the four types of reactions. When the conjugate acid and the conjugate base are of unequal strengths, the solution can be either acidic or basic, depending on the relative strengths of the two conjugates. Occasionally the weak acid and the weak base will have the same strength, so their respective conjugate base and acid will have the same strength, and the solution will be neutral. To predict whether a particular combination will be acidic, basic or neutral, tabulated K values of the conjugates must be compared.
Neutralization Reactions
This is the general format for a neutralization reaction:
Acid + Base → Salt + Water
It is important to note that neutralization reactions are just a specific type of double displacement redox reaction . Remember the rules for writing displacement reactions.
- Figure out what the reactants and products will be.
- The cations will switch places in the products for double replacement reactions.
- The element will replace the cation in the reacting compound and result in a new product for single replacement reactions.
- Make sure that all of the compound formulas are correctly written based on the oxidation state of the elements involved.
- Balance the equation.
Example \(\PageIndex{6}\): Predicting the outcome of a neutralization reaction
Write the balanced chemical equation for the neutralization of HCl with Mg(OH)2. Hint: neutralization reactions are a specialized type of double replacement reaction.
2HCl + Mg(OH)2 → Mg(Cl)2 + 2H2O
Since HCl is a strong acid and Mg(OH)2 is a strong base, the resulting solution would be neutral.
Stomach Antacids
Our stomachs contain a solution of roughly 0.03 M HCl, which helps us digest the food we eat. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful. When we have heartburn, it feels better if we reduce the excess acid in the esophagus by taking an antacid. As you may have guessed, antacids are bases. One of the most common antacids is calcium carbonate, CaCO3. The reaction,
\[CaCO_3(s)+2HCl(aq)⇌CaCl_2(aq)+H_2O(l)+CO_2(g)\]
not only neutralizes stomach acid, it also produces CO2(g), which may result in a satisfying belch.
Milk of Magnesia is a suspension of the sparingly soluble base magnesium hydroxide, Mg(OH)2. It works according to the reaction:
\[Mg(OH)_2(s)⇌Mg^{2+}(aq)+2OH^-(aq)\]
The hydroxide ions generated in this equilibrium then go on to react with the hydronium ions from the stomach acid, so that :
\[H_3O^+ + OH^- ⇌ 2H_2O(l)\]
This reaction does not produce carbon dioxide, but magnesium-containing antacids can have a laxative effect. Several antacids have aluminum hydroxide, Al(OH)3, as an active ingredient. The aluminum hydroxide tends to cause constipation, and some antacids use aluminum hydroxide in concert with magnesium hydroxide to balance the side effects of the two substances.
Culinary Aspects of Chemistry
Cooking is essentially synthetic chemistry that happens to be safe to eat. There are a number of examples of acid-base chemistry in the culinary world. One example is the use of baking soda, or sodium bicarbonate in baking. NaHCO3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
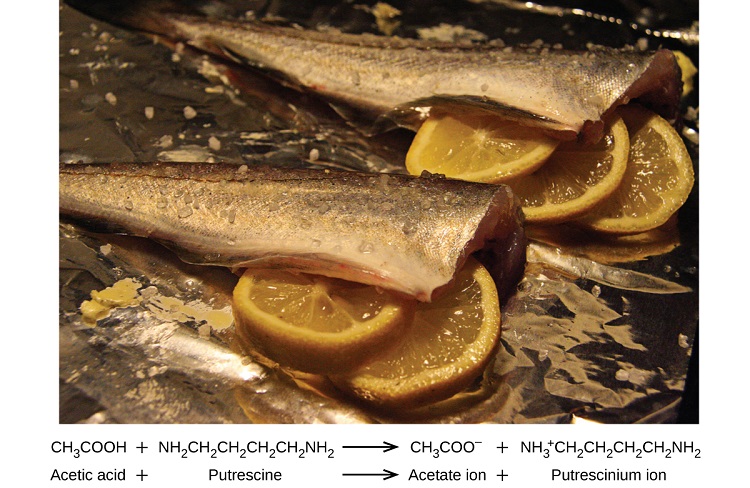
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish (Figure \(\PageIndex{1}\)). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odor of the fish, and also adds a “sour” taste that we seem to enjoy.
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favors the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavor of the vegetables with the acid making them taste sour.
Summary
The characteristic properties of aqueous solutions of Brønsted-Lowry acids are due to the presence of hydronium ions; those of aqueous solutions of Brønsted-Lowry bases are due to the presence of hydroxide ions. The neutralization that occurs when aqueous solutions of acids and bases are combined results from the reaction of the hydronium and hydroxide ions to form water. Some salts formed in neutralization reactions may make the product solutions slightly acidic or slightly basic.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).


