Many layered dichalcogenides, such as TiS2 and ZrS2, have the CdI2 structure. In these compounds, as we have noted above, the metal ions are octahedrally coordinated by S. Interestingly, the structures of MoS2 and WS2, while they are also layered, are different. In these cases, the metal is surrounded by a trigonal prism of sulfur atoms. NbS2, TaS2, MoSe2, MoTe2, and WSe2 also have the trigonal prismatic molybdenite structure, which is shown below alongside a platy crystal of MoS2.
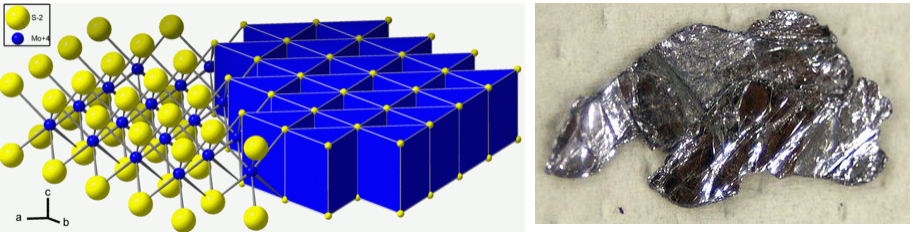
The coordination of the metal ions by a trigonal prism of chalcogenide ions is sterically unfavorable relative to octahedral coordination. There are close contacts between the chalcogenide ions, which are eclipsed in the stacking sequence AbA/BaB/AbA/BaB... (where "/" indicates the van der Waals gap between layers). What stabilizes this structure?
The molybdenite structure occurs most commonly in MX2 compounds with a d1 or d2 electron count. The figure below compares the splitting of d-orbital energies in the octahedral and trigonal prismatic coordination environments:
The trigonal prismatic structure is stabilized in MoS2 by filling the lowest energy band, the dz2. The dz2 orbital which points vertically through the triangular top and bottom faces of the trigonal prism, has the least interaction with the sulfide ligands and therefore the lowest energy. The dxz and dyz orbitals, which point at the ligands, have the highest energy. The dz2 orbital is lower in energy in this structure than the t2g orbitals are in the octahedral structure of TiS2.
|
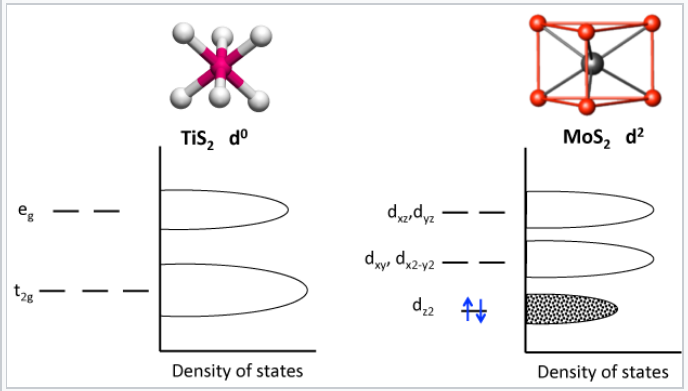
d-orbital splittings and energy bands in TiS2 and MoS2. MoS2 is a semiconductor with a 1.3 eV gap between its filled and empty bands.
|
Because it has an unfilled t2g band, TiS2 is relatively easy to reduce by intercalation with Li. For this reason, LiTiS2 was one of the first intercalation compounds studied by Stanley Whittingham, who developed the concept of the non-aqueous lithium ion battery in the early 1970's.[3] Because it has a filled dz2 band, MoS2 is harder to reduce, but it can be intercalated by reaction with the powerful reducing agent n-butyllithium to make LixMoS2 (x < 1). Atoms in the van der Waals planes of these compounds are relatively unreactive, which gives MoS2 its good oxidative stability and enables its application as a high temperature lubricant. Atoms at the edges of the crystals are however more reactive and in fact are catalytic. High surface area MoS2, which has a high density of exposed edge planes, is used as a hydrodesulfurization catalyst and is also of increasing interest as an electrocatalyst for the reduction of water to hydrogen.
Layered metal dichalcogenides, including MoS2, WS2, and SnS2, can form closed nanostructures that take the shape of multiwalled onions and multiwalled tubes. These materials were discovered by the group of Reshef Tenne in 1992, shortly after the discovery of carbon nanotubes. Since then nanotubes have been synthesized from many other materials, including vanadium and manganese oxides.
|
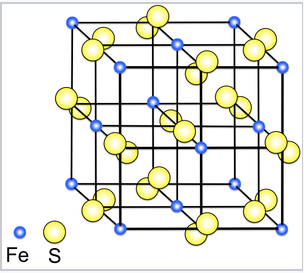
The pyrite (FeS2) crystal structure. The structure is related to NaCl, with Fe2+ and S22- ions occupying the cation and anion sites.
|
Although early (TiS2) and late (PtS2) transition metal disulfides have layered structures, a number of MS2 compounds in the middle of the transition series, such as MnS2, FeS2 and RuS2, have three-dimensionally bonded structures. For example, FeS2 has the pyrite structure, which is related to the NaCl structure. The reason is that FeS2 is not Fe4+(S2-)2, but is actually Fe2+(S22-), where S22- is the disulfide anion (which contains a single bond like the peroxide anion O22-). S2- is too strong a reducing agent to exist in the same compound with Fe4+, which is a strong oxidizing agent. Because FeS2 is actually Fe2+(S22-), it is a 1:1 compound and adopts a 1:1 structure.




