1.3: Periodic Properties of Atoms
- Last updated
- Save as PDF
- Page ID
- 344569
The Periodic Table of Elements
Having understood the Aufbau principle we can now understand the periodic table of the elements. The periodic table was actually invented a long time before the quantum-mechanical atom model was developed. It was first introduced by Mendeleev and Meyer who ordered the elements according to their masses in a row. Whenever an element had a property similar to an element that already was previously considered a new row was begun. This gave a table with rows and columns. Within a row the mass of the elements would increase, within a column one would find the elements that had similar properties. The rows would be called periods, and the columns would be called groups, hence the name periodic table of the elements. At the time Mendeleev and Meyer invented the periodic table, there were still many elements undiscovered visible as “holes” within the periodic table. However, over time the periodic table became complete (Fig. 1.3.1).
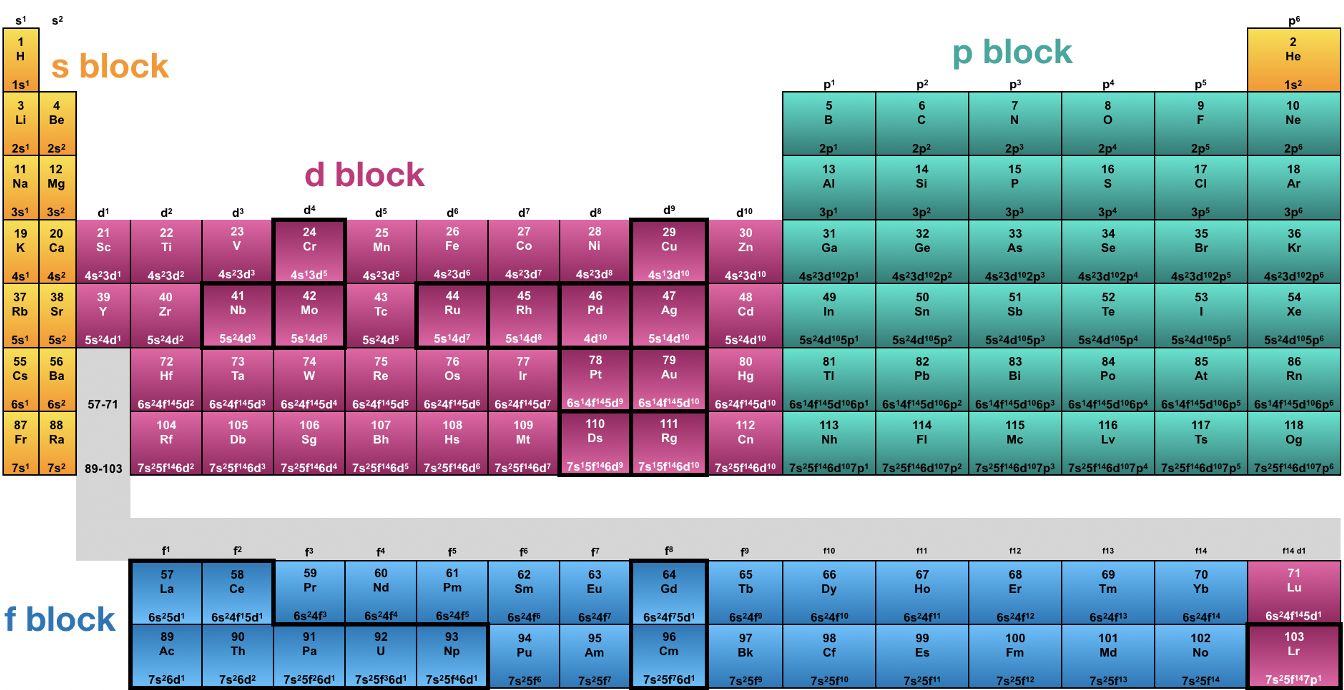
Today we know 118 elements completing seven periods and 18 groups. Today, the elements are not ordered by mass, but by the atomic number (number of protons), however this did not change the original periodic table much because except for a very few elements the mass of the elements follows the atomic number. Before the quantum-mechanical model was developed, it was not understood why elements within the same group had similar properties. When you analyze the electron configuration of the atoms you can see that all atoms in the same group have the same type of outmost electrons, also called valence electrons. These electrons are the electrons that have the greatest distance from the nucleus and have the highest energies. Because they have the highest energies, they are also the electrons that are most reactive, and determine the chemical and physical properties of the element. For example, in the first group all elements have one s electron as the valence electron. Because these electrons have the highest energy and are most reactive, they determine the properties of the atom, and thus the elements in the first group have similar properties.
When you look into group 2, all the electrons have two s electrons as the valence electrons. An exception is the element helium which is placed into group 18 which is called the group of the noble gases. The other group 18 elements have two s and six p electrons of the same quantum number n as valence electrons. The behavior of He is much more similar to that of the other group 18 elements, namely it is a gas with extremely low reactivity. This property is due to the fact that like the other noble gases He has a full shell. Elements with a full shell are particularly unreactive because full shells represent particularly stable electron configurations, also called noble gas configurations.
Group 1, group 2 and He are called the s-block of the periodic table because they are the elements that only have s electrons as valence electrons. From group 3 to group 12 there are elements with s and d electrons as valence electrons. These elements are the d-block of the periodic table. The d-block begins with group 3 where there is only one d electron together with two s electrons, and ends with group 12 where there are ten d and two s electrons. In group 12 the d subshell is completely filled with electrons. From group 13 to group 18 we find electrons that have p electrons as valence electrons. There are six groups because p orbitals can accommodate up to six p electrons. In group 13 there is only one p valence electron, in group 14 there are two, in group 15 there are three, in group 16 four, in group 17 five, and in group 18 there a six except the Helium which we previously discussed already. Group 13 to 18 is called the p block of the periodic table. The s-block and the p block elements together are also called the main group elements, the elements of the d-block are called the transition metals. The elements of the main groups have specific names. Group 1 elements except hydrogen are called the alkali metals, group 2 elements the earth alkaline metals, group 13 elements the triels, group 14 elements the tetrels, group 15 elements the pnictides, group 16 elements are the chalcogens, group 17 elements the halogens, and group 18 elements the noble gases.
Finally, there is the f block of the periodic table. The f-block contains the elements with f-valence electrons. Because there are seven f-orbitals for a given quantum number n, the f orbitals can accommodate up to 14 electrons, and thus there are 14 groups. The f block is actually located in between the s block and the d block of the periodic table, but is typically written underneath the rest of the periodic table. This is done because the periodic table would become too wide if it was placed between the s and the d block. Note that the f-block elements do not have a group number. Note also that the elements on the left end of the f-block, lanthanum and actinium, actually do not have f-valence electrons. They are actually group 3 elements located underneath the elements scandium and yttrium. The first row of the f-block is are called the lanthanides, which is the greek meaning the elements following the lanthanum. Only the elements following the lanthanum have f-valence electrons. Analogously, the actinium is not an actual f-block element, only the elements that follow the actinium. They are called the actinides.
The quantum mechanical model of the atoms also explains why the periodic table has periods. Each period is associated with a quantum number n, the first period with n=1, second period with n=2 and so forth. Within a period the available s and p valence orbitals of a specific quantum number n are getting filled. Subshells of a lower quantum number may also be filled within the period. For example, the 3d subshell gets filled within the fourth period, and the 4f subshell gets filled in the 6th period.
Elements with electron configurations that deviate from the expected electron configuration are highlighted in bold (Figure 1.3.1). There are only exceptions for the d-block and f-block elements. We discussed some of them already, for example the Cr and the Cu. In addition, for instance, Pd has the electron configuration 4d10 instead of 5s24d8, and Pt has the electron configuration 6s15d9 instead of 6s25d8. We can explain these exceptions when considering the complex electrostatic and magnetic interactions of electrons in multi-electron atoms. Not everything can be explained by the simple concepts of Slater rules and Hund’s rule.
As chemists we should always know our periodic table. Learning is easier with music, and there is a fabulous song by Tom Lehrer that can help you to learn the periodic table. The link to a respective YouTube video is provided below.
Atomic Radii
Let us now look at what is called the periodic properties of atoms. These are properties which change due to the position of the element within the periodic table. Let us first consider how the atomic radii of the atoms depend on the position of the atom in the periodic table, Fig. 1.3.2.
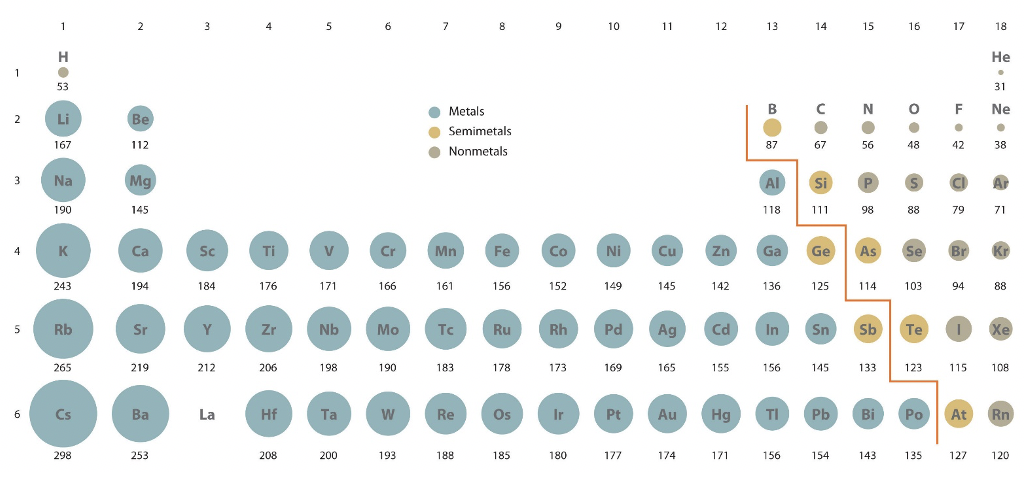
When you look at how the atomic radii change within a period you can easily see that they decrease from the left to the right in the periodic table. The decrease is more significant for the s and p block elements compared to the d block elements. We can explain this effect using the concept of effective nuclear charge. As we go from the left to the right in the periodic table we add electrons to the same shell. Because also a proton is added to the nucleus with each electron added, the electron experiences a greater attractive force from the nucleus. This effect tends to increase the effective nuclear charge. However, there is also an opposite effect. Because there is one more electron, there are more electron-electron repulsion and shielding effects, and this tends to decrease the effective nuclear charge. Because the new electron is added to the same shell, the shielding effects do not fully compensate for the additional Coulomb force coming from the additional proton in the nucleus, and thus the effective nuclear charge increases. This higher effective nuclear charge pulls the electrons closer to the nucleus, and thus the atom size decreases. For the d-block elements new d electrons get added to a more inner subshell, for example for the d-block elements of the 4th period the d electrons get added to the 3d subshell. Because of that, these 3d electrons can more effectively shield nuclear charge from the outmost two 4s electrons, and thus the decrease of the atomic radius is less pronounced. In summary:
Trend 1: Atomic radius decreases continuously within a period.
A second trend is that each time a new period is begun the atomic radius jumps dramatically. For example it jumps from 31 pm to 167 pm as we go from the helium to the lithium, and from 38 pm to 190 pm as you go from the neon to the sodium. This trend is because the electrons get filled into a new, more outer shell when a new period is begun. This outer shell is located much farther away from the nucleus, and thus the radius of the atom increases. In addition, the electrons in the inner shells can very effectively shield the nuclear charge from the electron added to the new shell which tends to increase atom radius. In summary:
Trend 2: Atomic radius jumps when a new period is begun.
As a third trend you can see that the atomic radii increase as you go down a group. This is because within a group there are the same number and type of valence electrons, but the valence electrons are in a more outer shell associated with a higher quantum number n. In a somewhat different view we can also argue that the first two trends add up to give the third trend. The effect of jump of atomic radius when a new period is begun is greater than the effect of decline of the radius within a period. As a result, the radius tends to increase as you go down a group. In summary:
Trend 3: Atomic radius increases continuously within a group.
The determination of an atomic radius is not as trivial at it may seem. You can obtain it either from quantum mechanical calculations, or determine it experimentally. If you calculate it, then the radius is defined as the radius that defines the volume in which the electron can be observed with 90% probability. The radii that you see in Figure 1.3.2 are actually calculated radii. If you determine the radius experimentally, then there are again different subtypes of radii. The so-called metallic, or crystal radii, the vanderWaals radii, and the covalent radii. The covalent radius is the half of the distance between two, same atoms in a molecule held together in a single covalent bond.
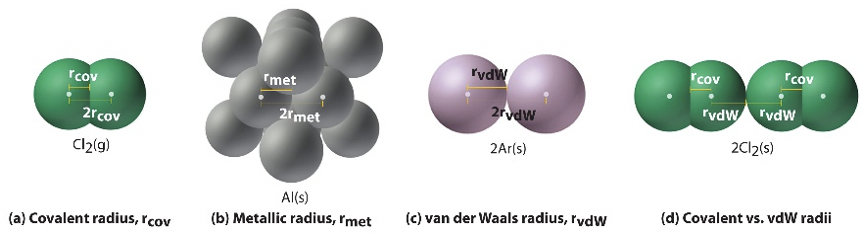
This is unambiguous for molecules such as Cl2, the other halogens, and for other cases such as hydrogen, carbon (as diamond), sulfur, and a few other cases. However for oxygen, O2, the situation is less clear as the order of the oxygen-oxygen bond is 2. In this case, it is necessary to infer the covalent radius from molecules containing O-O single bonds or from molecules containing an E-X single bond (E=element) in which the covalent radius of X is known. The van der Waals radius is half the distance between two same atoms in a crystal when there is no actual bonding between the atom except weak van der Waals forces. For example, in solid argon, there are only van der Waals forces between the atoms, and half the distance between the atoms in solid argon is the Van der Waals radius. For chlorine, for example, the van der Waals radius is half the distance between two chlorine atoms of two different Cl2 molecules in solid Cl2. Note that the van der Waals radius of chlorine is different than the covalent radius of chlorine. This again shows that there is a certain ambiguity when determining an atomic radius, and therefore it is always important to say what definition of atomic radius you use. Lastly, there is also the metallic radius that applies to solid metals in which metal atoms are held together by metallic bonding. The metallic radius is the defined as half the distance between the centers of two metal atoms within a metal.
First Ionization Energy
The first ionization energy is another important periodic property of the elements. It is defined as the energy required to remove an electron from a neutral atom in the gas phase. It is important to understand that we refer to an atom in the gas phase here, because only in the gas phase atoms do not significantly interact with each other. Therefore, only when looking at an atom in the gas phase we can truly determine the properties of single, isolated atoms. Let us look at the two images (Figure 1.3.4) to determine periodic trends.
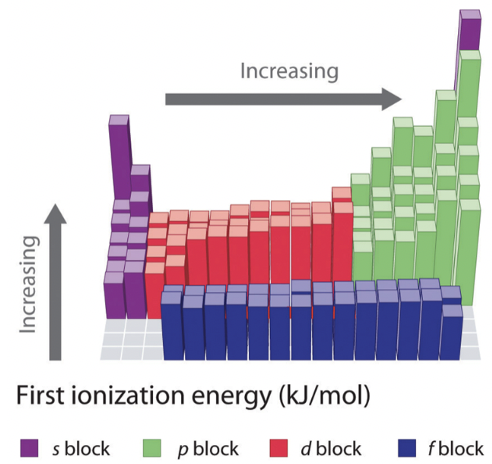
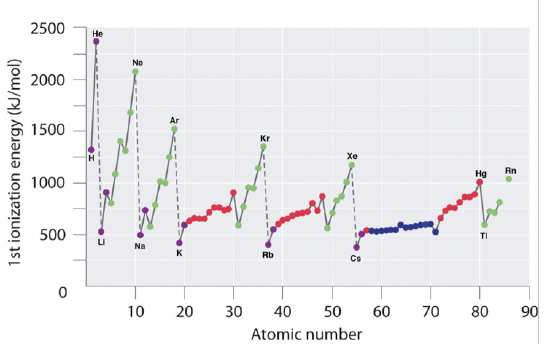
First of all we need to realize that all 1st ionization energies are positive. This means that it always requires energy to remove an electron from a neutral atom. You can understand this when you consider that all electrons in an atom are bound to the nucleus via a binding energy. If an ionization energy was negative then this would mean that the atom would spontaneously lose an electron, and spontaneously ionize. This would be unreasonable.
Now let us see how the first ionization energy changes within a period. We can see that it increases as we go from the left to the right within a period. This trend can be explained by the fact that the effective nuclear charge increases from the left to the right within a period. As the effective nuclear charge increases, the pull of the nucleus on the outmost electron increases, and thus the harder it is to remove this electron from the atom. You can see, however, that within the main group elements the trend is two times broken. The group 13 elements have a lower ionization energy than the group 2 elements, and the group 16 elements have a lower ionization energy than the group 15 elements. This phenomenon can be explained by the fact that the filled and half-filled subshells in group 2 and group 15 represent particularly stable electron configurations, and thus the electrons within these subshells have unusually low energy. For that reason these electrons are harder to remove from the atoms. The group 2 elements have filled s subshells, and the group 15 elements have half-filled p subshells. You can also notice that the increase in ionization energy is less pronounced within the d-block, and even less pronounced with in the f-block. This is because the effective nuclear charge on the outmost electrons does not increase as much because inner d and f orbitals are getting filled. You can also see that the group 13 elements have a lower ionization energy than the group 12 elements. This is because in group 13 a new p subshell is begun and the new electrons get added to the outmost shell where shielding effects are smaller.
As a second trend you can see that whenever a new period is begun, the ionization energy drastically drops. This is because the new electron is added to a new, more outer shell where the effective nuclear charge acting on the new electron is much smaller. The third trend is that the ionization energy becomes smaller as we go down a group. This is because the effective nuclear charge on the valence electrons decreases because the quantum number n of the valence electrons increases. There is one peculiarity that needs additional explanation. The drop of ionization energy from hydrogen to lithium in the first group is unusually large. This is because hydrogen is the only element for which there are no shielding effects, simply because the hydrogen has only one electron. For that reason the valence 1s electron of the hydrogen is far harder to remove than the valence s electrons of the other group 1 elements.
Electron Affinity
Now let us consider the first electron affinity (Figure 1.3.5). It is defined as the energy required or released when you add an electron to a neutral atom in the gas phase. Again, we look at an atom in the gas phase, because we want to consider an isolated atom that does not make significant interactions with other atoms.
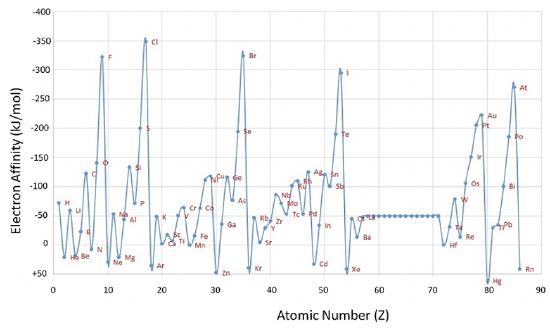
We can see from the graph that most elements have negative electron affinities, meaning that the addition of a free electron to a neutral atom is exothermic, and releases energy, however, this is not always the case. Some elements have negative electron affinities, in particular the noble gases, but also Be, N, Mg, Zn, Cd, and Hg. Zn, Cd, and Hg, are group 12 elements with a full d subshell which is particularly stable. An additional electron would need to be added to a p orbital of a higher shell which is energetically quite unfavorable. Similarly, Be and Mg have filled s subshells which are also fairly stable. Adding an additional electron would need to start an new subshell, which is energetically not favorable. You can see that Ca and Sr have electron affinities of about 0, and that of Ba is only slightly negative showing that higher periods make the addition of an electron to a new p shell slightly more favorable. Nitrogen has a half-filled p subshell, which is also a quite stable electron configuration, and therefore adding an electron is not favorable. The addition of an electron is slightly more favorable for the other group 15 elements, however, these also tend to have rather low electron affinities.
Generally, for the main group elements the electron affinity tends to increase from the group 1 to group 17. However, the trend is broken for group 2 and group 15 elements because these elements have full and half-filled subshells, respectively. In addition, it is noteworthy that group 1 elements have higher electron affinities than group 3 elements. This is because adding an electron to a group 1 element produces a full s subshell which is fairly stable. For the d-block elements, the electron affinity tends to increase from group 3 to group 11, but the trend is broken multiple times showing that each element would need to be investigated individually which is beyond the scope here. There is a big drop in electron affinity from group 17 to group 18 which is easily explained by the fact that the addition of an electron to a group 17 element produces a filled shell, while a group 18 element already has a full shell, and the addition of an electron would start a new shell. Similarly, there is a sharp drop in electron affinity from group 11 to group 12, as an addition of an electron to a group 11 atom produces a full subshell, while the addition of an electron to a group 12 element would require the start of new, more outer subshell. For the p block elements the electron affinity tends to first increase from period 2 to 3, and then decrease. For the s-block there is a steady decrease down a group. For d block elements there is no clear trend for the electron affinity.
Electronegativity
Lastly, let us study the electronegativity as a periodic property. The electronegativity is the measure of the ability of an atom to attract electrons within a chemical bond. It is related to the electron affinity in the sense that both period properties measure the force by which an electron is attracted to a neutral atom. However, the electron affinity is associated with isolated atoms, that do not make bonds to neighbored atoms, while the electronegativity refers to atoms that are bonded to neighbored atoms. The importance of the electronegativity stems largely from its ability to predict and understand the nature of the bonding between atoms, in particular, covalent, ionic, and metallic bonding.
Electronegativity Scales
Electronegativity was a concept first developed by Linus Pauling to describe the relative polarity of bonds and molecules. He argued that the electronegativity of an atom could be derived from bond energy differences between homoleptic and heteroleptic bonds (Eq. 1.3.1).

Equation 1.3.1 Electronegativity according to Pauling, eV = electron volts.
For example, the energy required to break the bond in H2 is 432 kJ mol-1, and for F2 it is 159 kJ mol-1. However the energy required to break a bond in HF is 565 kJ mol-1, which is much higher than expected just by averaging the energy of the two homonuclear bonds (296 kJ mol-1). Pauling argued that the difference could be assigned to electrostatic attractions between the F and H “ends” of the molecule whereby the F end has more electron density and the H end has less electron density. The greater the difference between the average energy of the homoleptic bonds and the heteroleptic bonds, the greater the electronegativity difference between the atoms, and the greater the polarity of the heteroleptic bond.
First, Pauling used the arithmetic means of the heteroleptic bond energies, later he used the geometric means, because he found empirically that it worked better (Equation 1.3.1). The geometric mean of the homoleptic bond energies is the square root of the product of the homoleptic bond energies. This method only provided electronegativity differences. He therefore needed to define a reference atom with an arbitrarily defined electronegativity value, and then determine the electronegativity values of all other atoms relative to that value. He chose the most electronegative atom, the fluorine atom, as the standard and assigned a value of 4.0. The values of all other atoms vary between 0.7 (Fr) and 3.44 (O), and are shown in the periodic table on the below (Fig. 1.3.6).
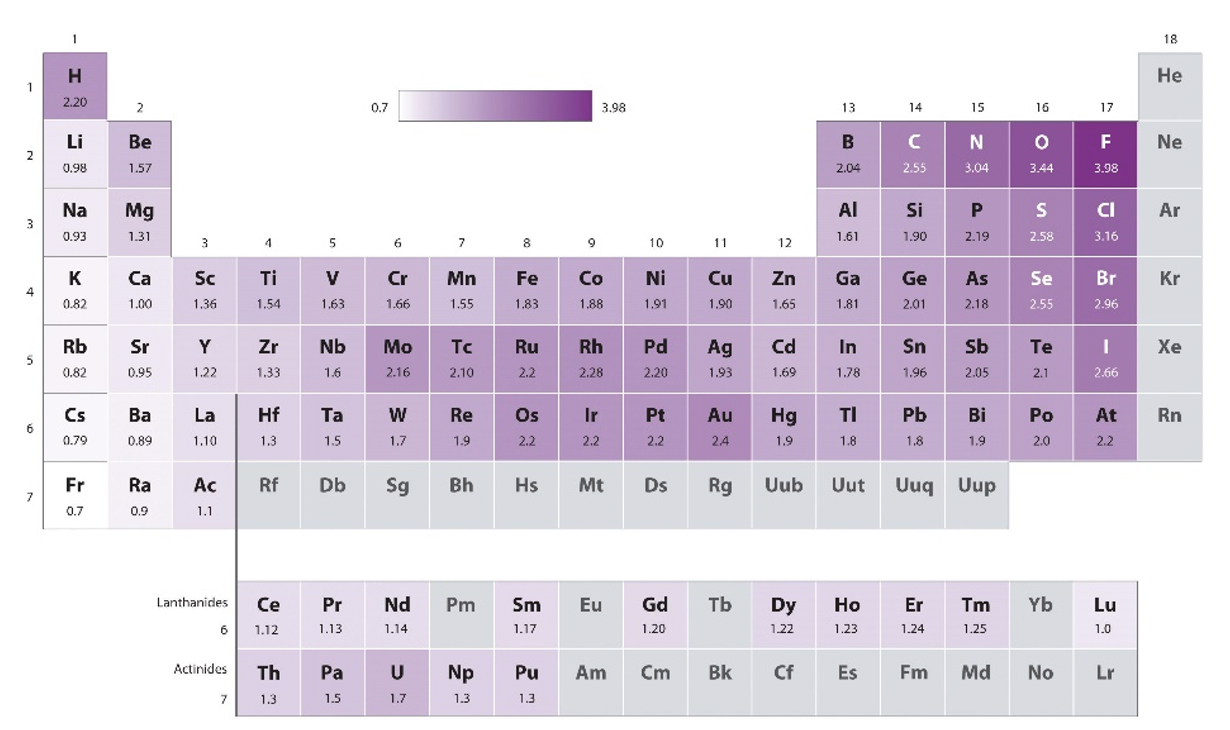
What are the periodic trends? One can see that within a period the electronegativity values of the main group elements strictly increase with the group number. Note though that the noble gases do not have a Pauling electronegativity because, with few exceptions, noble gases do not make compounds and thus no bond energies are available for them. We can also see that for main group elements the electronegativity strictly decreases down a group. This makes the fluorine the most electronegative atom, and the cesium the least electronegative atom. Cesium has an electronegativity value of 0.7. We ignore the radioactive francium here. For the d-block, the trends are less strict, but there is a tendency of electronegativity increase from group 3 to group 11. All group 12 elements have smaller electronegativities compared to their neighbored group 11 elements. What about trends within a group? In group 3 to group 5 there is a small decrease in electronegativity down a group. For group 6 and 9, the period 5 elements have higher values than their neighbored elements in period 4 and 6. For group 10 to 12 the electronegativity increases down a group. The d-block element with the highest electronegativity is the gold, it has a value of 2.4. Note that this is similar to the electronegativity of non-metals such as I, S, and P. For these reasons, sometimes gold can behave like a non-metal in compounds with very low electronegativity. For example, there is the compound cesium auride (CsAu) which is a transparent, ionic crystalline compound due to the high electronegativity difference between cesium and gold. The f-block elements generally have low electronegativity.
The Pauling electronegativity scale is derived from empirical data on bond energies. It works very well in practice and it is up to date the most used electronegativity scale. However, it does not to relate electronegativity to other periodic properties, and the quantum-mechanical model of the atoms. One electronegativity scale that addresses this short-coming the Allred-Rochow scale. It relates the electronegativity to the Coulomb force that acts on an electron on the surface of an atom. The Coulomb force is proportional to the effective nuclear charge Z* and inverse proportional to the atomic radius square. Z*/r2 is multiplied with a factor of 3590 and a value of 0.744 is added to this term. These numbers are empirically chosen so that the values of the Allred-Rochow scale become comparable to the Pauling scale.
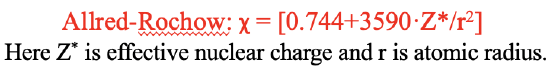
Equation 1.3.2 Allred-Rochow definition of electronegativity
Another frequently used scale is the Mulliken electronegativity scale. The Mulliken scale relates the electronegativity to the sum of the first ionization energy IE and the first electron affinity EA. We would intuitively agree that the ability of an atom to attract electrons within a chemical bond is the higher, the harder it is to remove an electron from an isolated atom, and the easier it is to add an electron to an isolated atom. The numbers 0.118 and -0.207 are empirically chosen to make comparisons to the Pauling scale possible. The Mulliken scale is related to the Allred-Rochow scale in the sense that you can explain ionization energies and electron affinities of atoms with the concept of the effective nuclear charge.

Equation 1.3.3 Mulliken definition of electronegativity
Another electronegativity scale has been developed by Leland C. Allen. It designed for main group elements in particular. It argues that the electronegativity is proportional to the average energy of the s and p valence electrons in the atom. This electronegativity concept also has a relationship to that of Allred-Rochow, because orbital energies can be calculated from the effective nuclear charge.
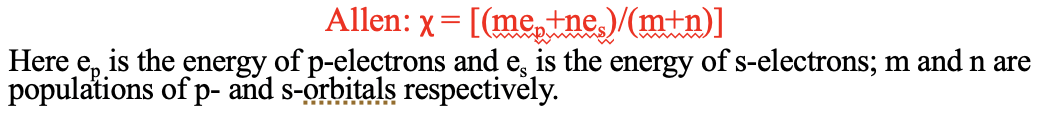
Equation 1.3.4 Leland C. Allen definition of electronegativity
Overall, you can see that the electronegativity scales are inter-related, they say essentially the same, but present electronegativity from a somewhat different perspective.
Electronegativity of the Elements and Chemical Bonding
One of the most powerful attributes of electronegativity is that it can predict what type of chemical bond is to expect in elements and compounds. Let us first look at the bonding between elements.
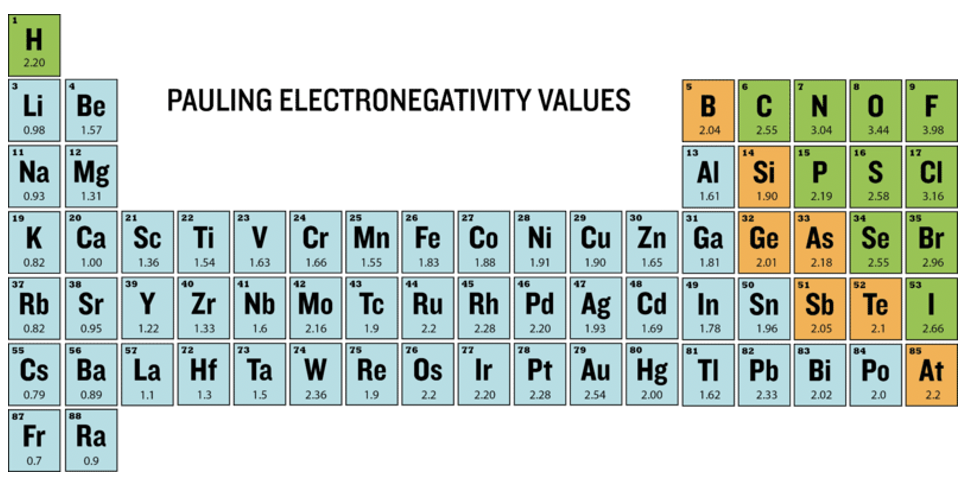
In the depicted periodic table (Figure 1.3.7) you can see the type of chemical bonding between atoms indicated by different colors. There is metallic bonding in metals, indicated by the color blue. In metallic bonds the electrons are shared between atoms but are delocalized over many atoms in the metal. The green color indicates covalent bonding seen in non-metals. In covalent bonding the electrons are shared, but localized in between typically two atoms. Metalloids have chemical bonding with a mix of covalent and metallic character shown in orange. There is electron-sharing with a moderate degree of delocalization. The Pauling electronegativity values of the elements are written underneath the element symbols.
Can we relate the electronegativity values to the chemical bonding in the elements? We can clearly see that elements with high electronegativity values significantly above 2.0, the non-metals, tend to make covalent bonds in between them. All metalloids with a hybrid covalent-metallic character, have intermediate electronegativity around 2.0. Most metals have low electronegativity values below 2.0 except noble metals like Pt and Au. Overall, we can say that there is a clear relationship between the bonding type in an element and its electronegativity. Now let us see if electronegativity can also predict chemical bonding in compounds.
Ketelaar's Triangle
The ability of electronegativity to predict the bonding type in compounds can be understood by Ketelaar’s triangle, named after J.A.A. Ketelaar.
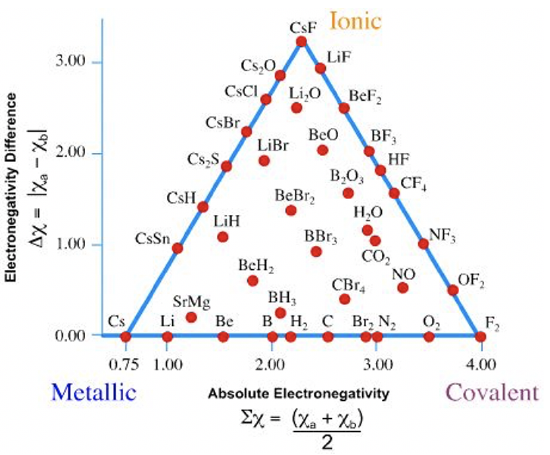
You can see that Ketelaar’s triangle has three corners (Figure 1.3.8). Two of the corners are occupied by the elements F and Cs, and the third corner is occupied by CsF. All elements are located on the horizontal edge of the triangle, and there many compound indicated by dots either on the two other edges or within the triangle. There are no compounds or elements outside of the triangle. How can we understand this triangle and what does it say about the relationship between bonding character and electronegativity? To understand this, look at the two axes. The horizontal x-axis represents the average electronegativity of the atoms in an element or a compound. The vertical y-axis represents the electronegativity difference between the atoms in the elements or the compound. For elements the electronegativity difference between the atoms is zero, because in an element all atoms are of the same type. Therefore, all the elements lie on the horizontally oriented edge of the triangle. Because Cs is the element with the lowest electronegativity, it lies furthest to the left on this edge. Fluorine is the element with the highest electronegativity, and thus lies the furthest on the right side. The other elements are in located in between the cesium and the fluorine on the edge. The higher the electronegativity of the elements, the further right their position on this edge of the triangle.
For compounds, the electronegativity difference between atoms is never exactly zero, therefore all compounds are located above the horizontal edge of the triangle. The compound with the highest electronegativity difference is the CsF. Its average electronegativity is the sum the electronegativity of Cs and F divided by 2: Therefore, CsF defines the third corner of the triangle. All other compounds must lie on the edges or within the triangle. All cesium compounds are located on the edge between the Cs and CsF, and all the fluorine compounds are located on the edge between CsF and F2. The compounds of all other elements are inside the triangle. The position of the compound on or in the triangle defines its bonding character. The closer the position of the compound toward the Cs corner, the more metallic, the closer the position is to the F2 corner, the more covalent, and the closer it is to the CsF corner, the more ionic. For example Li2O is located close to CsF, and the bonding would be predominantly ionic, although there is also a small degree of covalent and metallic bonding. In contrast to that, SrMg has mostly metallic bonding, a little bit of covalent bonding, and even less ionic bonding. The bonding situation in LiH is in between metallic and ionic, with a little bit of covalent bonding character mixed in. Note that a 100% ionic bond is not possible because the electronegativity difference is finite, and thus there must always be a certain degree of electron sharing. In contrast to that, a 100% covalent bond is possible because electrons can be equally shared between two atoms when the electronegativity difference is zero.
The take home message is that in compounds and elements there is usually a mix of bonding types, and not a single bonding type, even through one bonding type may strongly dominate. The concept of electronegativity, and Ketelaar’s triangle in particular, is extremely helpful to predict to which degree the three bonding types are present in a substance.
Dr. Kai Landskron (Lehigh University). If you like this textbook, please consider to make a donation to support the author's research at Lehigh University: Click Here to Donate.


