10.3: Molecular Definitions of Acids and Bases
- Last updated
- Save as PDF
- Page ID
- 212516
Learning Objectives
- Identify an Arrhenius acid and an Arrhenius base.
- Identify a Brønsted-Lowry acid and a Brønsted-Lowry base.
- Identify conjugate acid-base pairs in an acid-base reaction.
There are three major classifications of substances known as acids or bases. The theory developed by Svante Arrhenius in 1883, the Arrhenius definition, states that an acid produces H+ in solution and a base produces OH-. Later, two more sophisticated and general theories were proposed. These theories are the Brønsted-Lowry and Lewis definitions of acids and bases. This section will cover the Arrhenius and Brønsted-Lowry theories; the Lewis theory is discussed elsewhere.
The Arrhenius Theory of Acids and Bases
In 1884, the Swedish chemist Svante Arrhenius proposed two specific classifications of compounds, termed acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. An Arrhenius acid is a compound that increases the concentration of \(\ce{H^{+}}\) ions that are present when added to water. These H+ ions form the hydronium ion (H3O+) when they combine with water molecules. This process is represented in a chemical equation by adding H2O to the reactants side.
\[ \ce{HCl(aq) \rightarrow H^{+}(aq) + Cl^{-}(aq) } \nonumber \]
In this reaction, hydrochloric acid (\(HCl\)) dissociates completely into hydrogen (H+) and chlorine (Cl-) ions when dissolved in water, thereby releasing H+ ions into solution. Formation of the hydronium ion equation:
\[\ce{ HCl(aq) + H_2O(l) \rightarrow H_3O^{+}(aq) + Cl^{-}(aq)} \nonumber \]
An Arrhenius base is a compound that increases the concentration of \(\ce{OH^{-}}\) ions that are present when added to water. The dissociation is represented by the following equation:
\[\ce{ NaOH \; (aq) \rightarrow Na^{+} \; (aq) + OH^{-} \; (aq) } \nonumber \]
In this reaction, sodium hydroxide (NaOH) disassociates into sodium (\(\ce{Na^{+}}\)) and hydroxide (\(\ce{OH^{-}}\)) ions when dissolved in water, thereby releasing OH- ions into solution.
Arrhenius acids are substances which produce hydrogen ions in solution and Arrhenius bases are substances which produce hydroxide ions in solution.
Limitations to the Arrhenius Theory
The Arrhenius theory has many more limitations than the other two theories. The theory does not explain the weak base ammonia (NH3), which in the presence of water, releases hydroxide ions into solution, but does not contain OH- itself. The Arrhenius definition of acid and base is also limited to aqueous (i.e., water) solutions.
The Brønsted-Lowry Theory of Acids and Bases
In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases, ones that focus on proton transfer. A Brønsted-Lowry acid is any species that can donate a proton (H+) to another molecule. A Brønsted-Lowry base is any species that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor (PD), while a Brønsted-Lowry base is a proton acceptor (PA).
A Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
Let us use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
\[\ce{NH3(aq) + H2O (ℓ) <=> NH^{+}4(aq) + OH^{−}(aq) }\label{Eq1} \]
What has happened in this reaction is that the original water molecule has donated a hydrogen ion to the original ammonia molecule, which in turn has accepted the hydrogen ion. We can illustrate this as follows:

Because the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule—which accepts the hydrogen ion—is the Brønsted-Lowry base. Thus, ammonia acts as a base in both the Arrhenius sense and the Brønsted-Lowry sense.
Is an Arrhenius acid like hydrochloric acid still an acid in the Brønsted-Lowry sense? Yes, but it requires us to understand what really happens when HCl is dissolved in water. Recall that the hydrogen atom is a single proton surrounded by a single electron. To make the hydrogen ion, we remove the electron, leaving a bare proton. Do we really have bare protons floating around in aqueous solution? No, we do not. What really happens is that the H+ ion attaches itself to H2O to make H3O+, which is called the hydronium ion. For most purposes, H+ and H3O+ represent the same species, but writing H3O+ instead of H+ shows that we understand that there are no bare protons floating around in solution. Rather, these protons are actually attached to solvent molecules.
The Hydronium Ion
A proton in aqueous solution may be surrounded by more than one water molecule, leading to formulas like \(\ce{H5O2^{+}}\) or \(\ce{H9O4^{+}}\) rather than \(\ce{H3O^{+}}\). It is simpler, however, to use \(\ce{H3O^{+}}\) to represent the hydronium ion.
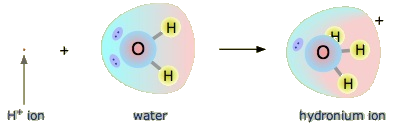
With this in mind, how do we define HCl as an acid in the Brønsted-Lowry sense? Consider what happens when HCl is dissolved in H2O:
\[\ce{HCl(g) + H_2O (ℓ) \rightarrow H_3O^{+}(aq) + Cl^{−}(aq) }\label{Eq2} \]
We can depict this process using Lewis electron dot diagrams:
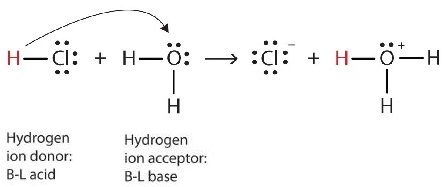
Now we see that a hydrogen ion is transferred from the HCl molecule to the H2O molecule to make chloride ions and hydronium ions. As the hydrogen ion donor, HCl acts as a Brønsted-Lowry acid; as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base. So HCl is an acid not just in the Arrhenius sense, but also in the Brønsted-Lowry sense. Moreover, by the Brønsted-Lowry definitions, H2O is a base in the formation of aqueous HCl. So the Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base—although the Arrhenius definition would not have labeled H2O a base in this circumstance.
- A Brønsted-Lowry acid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base is a proton (hydrogen ion) acceptor.
- All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. However, not all Brønsted-Lowry acids and bases are Arrhenius acids and bases.
Example \(\PageIndex{1}\)
Aniline (C6H5NH2) is slightly soluble in water. It has a nitrogen atom that can accept a hydrogen ion from a water molecule, just like the nitrogen atom in ammonia does. Write the chemical equation for this reaction and identify the Brønsted-Lowry acid and base.
Solution
C6H5NH2 and H2O are the reactants. When C6H5NH2 accepts a proton from H2O, it gains an extra H and a positive charge and leaves an OH− ion behind. The reaction is as follows:
\[\ce{C6H5NH2(aq) + H2O(ℓ) <=> C6H5NH3^{+}(aq) + OH^{−}(aq)} \nonumber \]
Because C6H5NH2 accepts a proton, it is the Brønsted-Lowry base. The H2O molecule, because it donates a proton, is the Brønsted-Lowry acid.
Exercise \(\PageIndex{1}\)
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
\[\ce{H2PO4^{-} + H_2O <=> HPO4^{2-} + H3O^{+}} \nonumber \]
- Answer
- Brønsted-Lowry acid: H2PO4-; Brønsted-Lowry base: H2O
Exercise \(\PageIndex{2}\)
Which of the following compounds is a Bronsted-Lowry base?
- HCl
- HPO42-
- H3PO4
- NH4+
- CH3NH3+
- Answer
-
A Brønsted-Lowry Base is a proton acceptor, which means it will take in an H+. This eliminates \(\ce{HCl}\), \(\ce{H3PO4}\), \(\ce{NH4^{+}}\) and \(\ce{CH_3NH_3^{+}}\) because they are Bronsted-Lowry acids. They all give away protons. In the case of \(\ce{HPO4^{2-}}\), consider the following equation:
\[\ce{HPO4^{2-} (aq) + H2O (l) \rightarrow PO4^{3-} (aq) + H3O^{+}(aq) } \nonumber \]
Here, it is clear that HPO42- is the acid since it donates a proton to water to make H3O+ and PO43-. Now consider the following equation:
\[ \ce{ HPO4^{2-}(aq) + H2O(l) \rightarrow H2PO4^{-} + OH^{-}(aq)} \nonumber \]
In this case, HPO42- is the base since it accepts a proton from water to form H2PO4- and OH-. Thus, HPO42- is an acid and base together, making it amphoteric.
Since HPO42- is the only compound from the options that can act as a base, the answer is (b) HPO42-.
Conjugate Acid-Base Pair
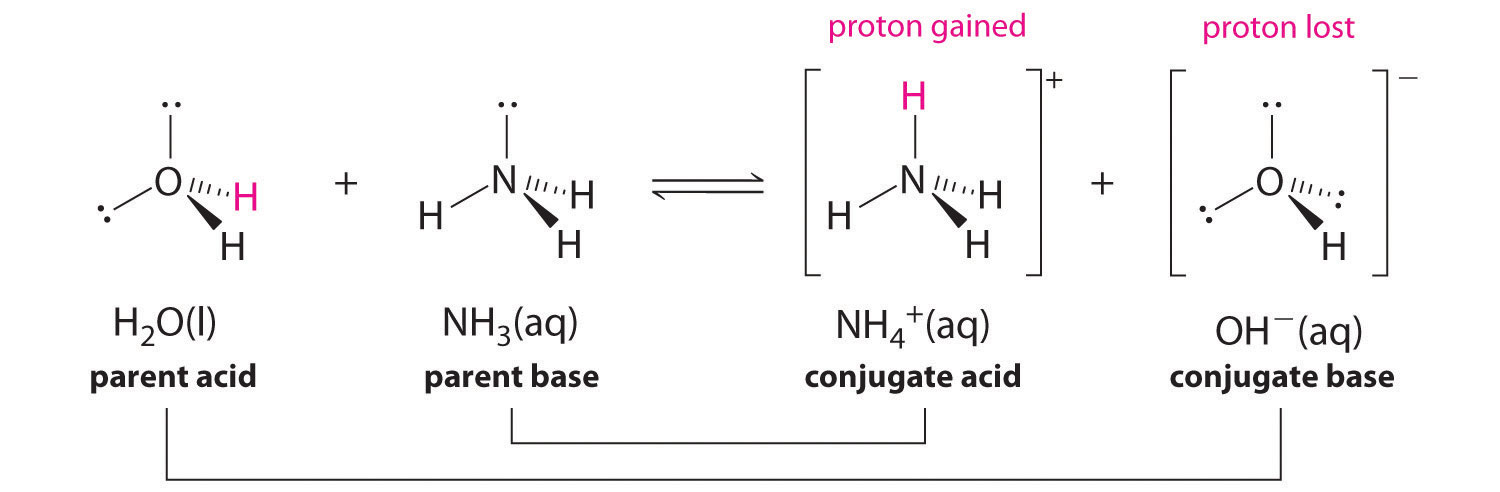
In reality, all acid-base reactions involve the transfer of protons between acids and bases. For example, consider the acid-base reaction that takes place when ammonia is dissolved in water. A water molecule (functioning as an acid) transfers a proton to an ammonia molecule (functioning as a base), yielding the conjugate base of water, \(\ce{OH^-}\), and the conjugate acid of ammonia, \(\ce{NH4+}\):
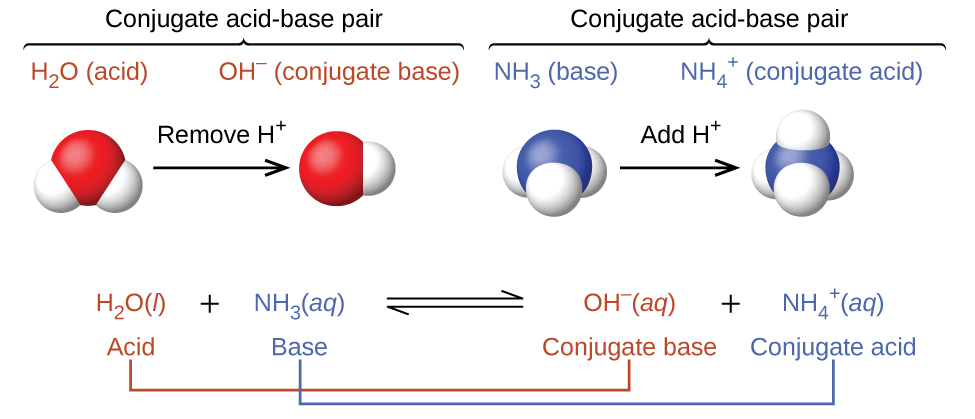
In the reaction of ammonia with water to give ammonium ions and hydroxide ions, ammonia acts as a base by accepting a proton from a water molecule, which in this case means that water is acting as an acid. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base. The conjugate acid–base pairs for this reaction are \(NH_4^+/NH_3\) and \(H_2O/OH^−\).
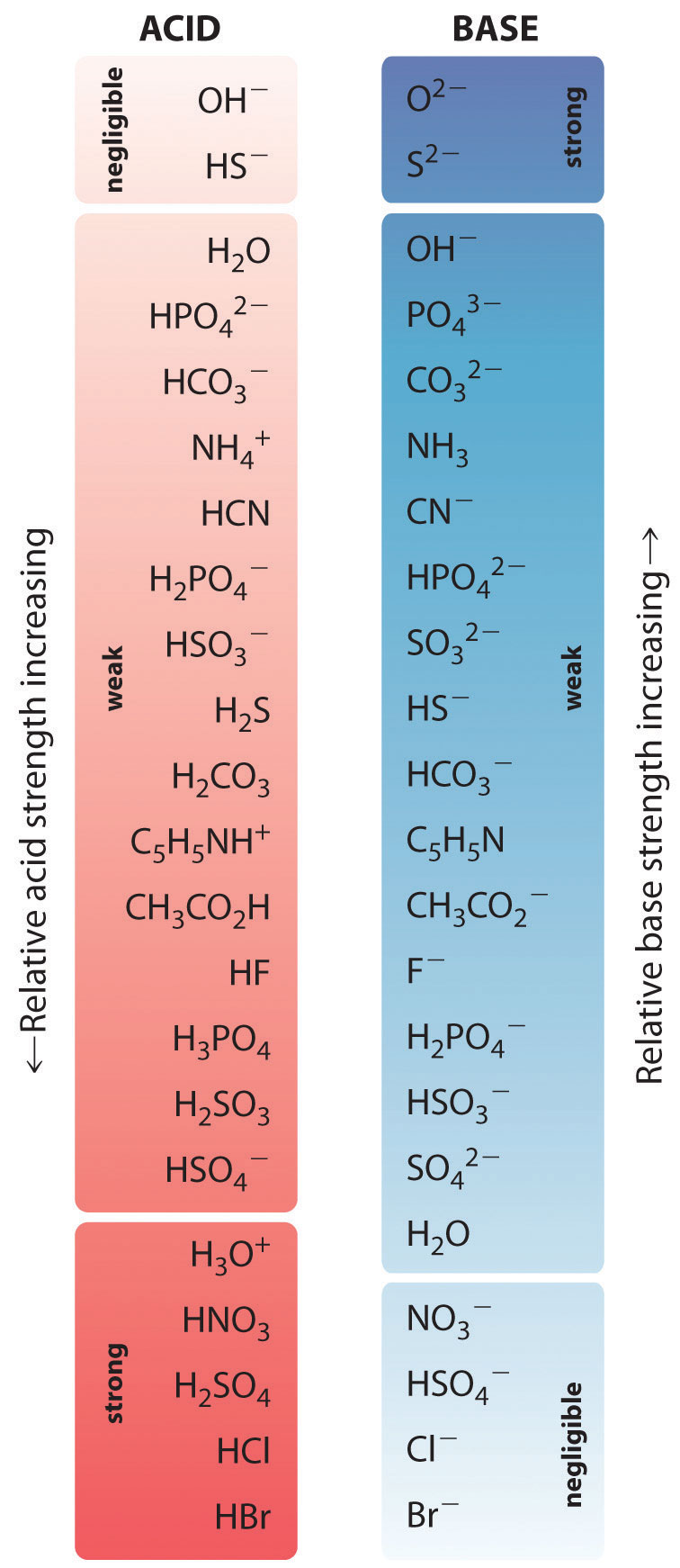
Example \(\PageIndex{2}\)
Identify the conjugate acid-base pairs in this equilibrium.
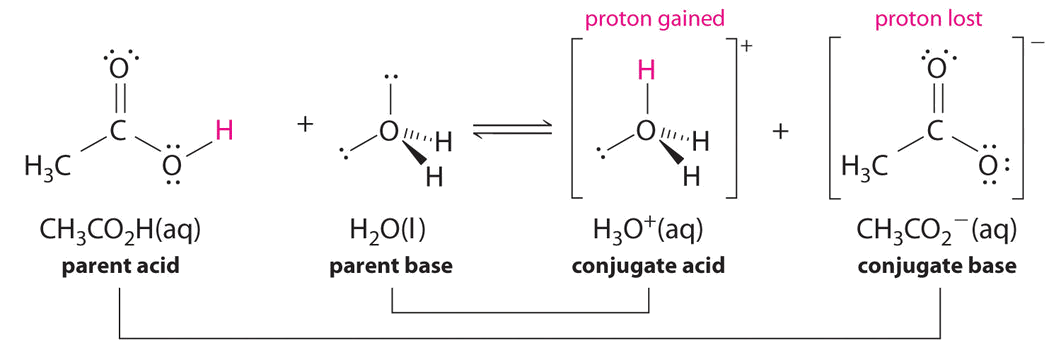
\[\ce{CH3CO2H + H2O <=> H3O^{+} + CH3CO2^{-}} \nonumber \]
Solution
Similarly, in the reaction of acetic acid with water, acetic acid donates a proton to water, which acts as the base. In the reverse reaction, \(H_3O^+\) is the acid that donates a proton to the acetate ion, which acts as the base.
Once again, we have two conjugate acid-base pairs:
- the parent acid and its conjugate base (\(CH_3CO_2H/CH_3CO_2^−\)) and
- the parent base and its conjugate acid (\(H_3O^+/H_2O\)).
Example \(\PageIndex{3}\)
Identify the conjugate acid-base pairs in this equilibrium.
\[\ce{(CH_{3})_{3}N + H_{2}O <=> (CH_{3})_{3}NH^{+} + OH^{-}} \nonumber \]
Solution
One pair is H2O and OH−, where H2O has one more H+ and is the conjugate acid, while OH− has one less H+ and is the conjugate base.
The other pair consists of (CH3)3N and (CH3)3NH+, where (CH3)3NH+ is the conjugate acid (it has an additional proton) and (CH3)3N is the conjugate base.
Exercise \(\PageIndex{3}\)
Identify the conjugate acid-base pairs in this equilibrium.
\[\ce{NH2^{-} + H2O\rightleftharpoons NH3 + OH^{-}} \nonumber \]
- Answer
- H2O (acid) and OH− (base); NH2− (base) and NH3 (acid)

