pH is measured on a scale of 1 – 14 with 7 being neutral; (see figure 1.) The lower the number the more acidic a solution is, the higher the number the more basic it is. What does acidic or basic mean? Acidity means there are more hydronium ions than hydroxide ions and vice versa for basic solutions. Why care about pH and pOH? All organisms live in certain pH and pOH levels. Your body tends to be between a pH of 7.35 and 7.45. When the pH is higher or lower than the preferred range, your body does not function well. If your body is acidic (lower than pH of 7,) you can develop problems such as inflammation and wearing away of body tissue. This can lead to cancer, heart disease, or diabetes. Your body prefers a slightly basic (higher than 7 pH) environment. But if it gets too high, it can cause digestive and respiration problems. There is a great balancing act happening. The same is happening in the Chesapeake Bay.
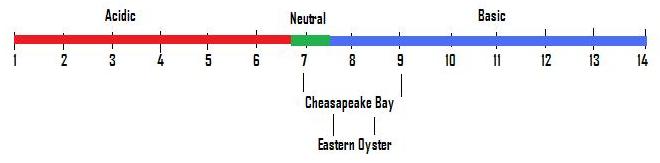
Figure \(\PageIndex{1}\): pH Scale
The Chesapeake tends to be between a pH of 7 and 9. When extra nutrients from farming (such as from fertilizers) leak into the water supply, algae can thrive and bloom (see Figure 2.) This causes an imbalance in the environment and creates a more alkaline (basic) pH water environment. A pH increase of 1 is more significant than it seems. pH is a log 10 scale, so a pH of 7 has a concentration of 10-7 hydronium ions. A pH of 8 has a concentration of 10-8. This means that a pH of 8 has 10 times less [one tenth] hydronium ions as a pH of 7 and a pH of 9 has 100 times less [one hundredth] hydronium ions than a pH of 7. So, there are consequences with just a change of 1 pH unit. To figure out the pH and pOH levels from ion concentration, use the following formulas. pH = -log (H3O+) and pOH = -log (OH-).

Figure \(\PageIndex{2}\): Algae Bloom http://drwaters.org/?itemCategory=33533&siteid=300&priorId=0
The Chesapeake Bay is home to the eastern oyster and other organisms. The eastern oyster can spawn in water with pH between 7.8 and 8.2 and had difficulties in pH that is lower than 6 or higher than 10. Larvae mortality rates increased rapidly when the pH is in the 9.00 to 9.50 range. The pH also affects respiration rate and pumping rate of water. When algae blooms occur, the oyster population suffers.

Figure \(\PageIndex{3}\): Eastern Oyster from the Chesapeake Bay http://www.chesapeakebay.net/fieldguide/critter/eastern_oyster
Practice Questions
Directions: Figure out the pH and pOH levels and answer the following questions.
1)What is the pH if the hydronium concentration is 10-5?
pH = -log (H3O+)
pH = -log (10-5)
pH = 5
2)Would the Eastern Oyster survive in this pH? Explain.
The Eastern Oyster population would not survive in a pH of 5. The Eastern oyster needs a pH of 7.8 – 8.2 to spawn and anything under a pH of 6 makes the respiration rate decrease with spawning nearly impossible. This means that the oyster population would not increase and the oysters that were there would die off slowly due to not being able to function properly in that low of a pH.
3)What would the pOH be if the hydroxide concentration is 10<su>-8</sup>?
pOH = -log (OH)
pOH = -log (10-8)
pOH = 8
4)If pOH = 14 – pH, what is the pH level from your answer to number 3?
pOH = 14 – pH
8 = 14 – pH
pH + 8 = 14
pH = 14 – 8
pH = 6
5)Would the Eastern Oyster survive in this pH? Explain.
It would be possible for the Eastern Oyster to survive since they have more difficulties when the pH is lower than 6. They would be stressed, but not to the point of being unable to reproduce. This is the lowest the pH could be before the population would really start to decline. This could potentially be the “tipping point” of the population.
From ChemPRIME: 14.2: pH and pOH
References
http://www.acidalkalinediet.com/alkaline-diet/how-to-control-your-ideal-body-ph
www.dnr.state.md.us/bay/monit...ter/index.html
www.dnr.state.md.us/irc/docs/00000260_03.pdf
Contributors and Attributions





