Oxidation
An important reaction of monosaccharides is the oxidation of the aldehyde group, one of the most easily oxidized organic functional groups. Aldehyde oxidation can be accomplished with any mild oxidizing agent, such as Tollens’ reagent or Benedict’s reagent. With the latter, complexed copper(II) ions are reduced to copper(I) ions that form a brick-red precipitate [copper(I) oxide;

Any carbohydrate capable of reducing either Tollens’ or Benedict’s reagents without first undergoing hydrolysis is said to be a reducing sugar. Because both the Tollens’ and Benedict’s reagents are basic solutions, ketoses (such as fructose) also give positive tests due to an equilibrium that exists between ketoses and aldoses in a reaction known as tautomerism.

Benedict’s Test. Benedict’s test was performed on three carbohydrates, depicted from left to right: fructose, glucose, and sucrose. The solution containing sucrose remains blue because sucrose is a nonreducing sugar

Tollen's test: left side positive (silver mirror, reducing sugar), right side negative (clear solution, non-reducing sugar). Image by FK1954, Public domain, via Wikimedia Commons
These reactions have been used as simple and rapid diagnostic tests for the presence of glucose in blood or urine. For example, Clinitest tablets, which are used to test for sugar in the urine, contain copper(II) ions and are based on Benedict’s test. A green color indicates very little sugar, whereas a brick-red color indicates sugar in excess of 2 g/100 mL of urine.
Ketoses are also reducing sugars due to an enol isomerism equilibrium. In this equilibrium, the ketone form is in equilibrium with the aldehyde form, which is responsible for the observed oxidation reaction:

Glycoside Formation
Being hemicacetal, monosaccharides in their cyclic form can react with a second alcohol molecule, forming an acetal. In this case, the creation can't be intramolecular due to steric hindrance. Acetal derivatives formed when a monosaccharide reacts with an alcohol in the presence of an acid catalyst are called glycosides.
This reaction is illustrated for glucose and methanol in the diagram below. In naming of glycosides, the "ose" suffix of the sugar name is replaced by "oside", and the alcohol group name is placed first. As is generally true for most acetals, glycoside formation involves the loss of an equivalent of water. The acetak product is stable to base and alkaline oxidants such as Tollen's reagent. Since acid-catalyzed formation of acetals is reversible, glycosides may be hydrolyzed back to their alcohol and sugar components by aqueous acid.
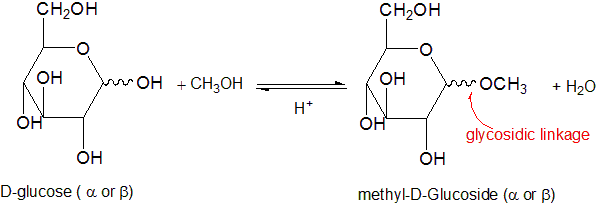
Glycosides abound in biological systems. By attaching a sugar moiety to a lipid or benzenoid structure, the solubility and other properties of the compound may be changed substantially. Because of the important modifying influence of such derivatization, numerous enzyme systems, known as glycosidases, have evolved for the attachment and removal of sugars from alcohols, phenols and amines. Chemists refer to the sugar component of natural glycosides as the glycon and the alcohol component as the aglycon.
Biological Ester Formation: Phosphorylation
Recall that almost all biomolecules are charged species, which 1) keeps them water soluble, and 2) prevents them from diffusing across lipid bilayer membranes. Although many biomolecules are ionized by virtue of negatively charged carboxylate and positively charged amino groups, the most common ionic group in biologically important organic compounds is phosphate - thus the phosphorylation of alcohol groups is a critical metabolic step. In alcohol phosphorylations, ATP is almost always the phosphate donor, and the mechanism is very consistent: the alcohol oxygen acts as a nucleophile, attacking the gamma-phosphorus of ATP and expelling ADP

