4.5: Factors affecting the SN2 Reaction
- Page ID
- 227558
Learning Objective
- determine the rate law & predict the mechanism based on its rate equation or reaction data for SN2 reactions
- predict the products and specify the reagents for SN2 reactions with stereochemistry
- propose mechanisms for SN2 reactions
- draw and interpret Reaction Energy Diagrams for SN2 reactions
Bimolecular nucleophilic substitution (SN2) reactions are concerted, meaning they are a one step process. The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen.
In order of decreasing importance, the factors impacting SN2 reaction pathways are
1) structure of the alkyl halide
2) strength of the nucleophile
3) stability of the leaving group
4) type of solvent.
Structure of the alkyl halide (Substrate) and SN2 Reaction Rates
Bimolecular nucleophilic substitution (SN2) reactions are concerted, meaning they are a one step process. The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen.
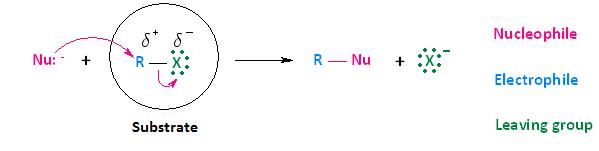
The SN2 transition state is very crowded with a total of five groups around the electrophilic center, the nucleophile, the leaving group, and three substituents.
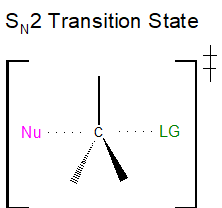
If each of the three substituents in this transition state were small hydrogen atoms, as illustrated in the first example below, there would be little steric repulsion between the incoming nucleophile and the electrophilic center, thereby increasing the ease at which the nucleophilic substitution reaction can occur. Remember, for the SN2 reaction to occur, the nucleophile must be able to overlap orbitals with the electrophilic carbon center, resulting in the expulsion of the leaving group. If one of the hydrogens, however, were replaced with an R group, such as a methyl or ethyl group, there would be an increase in steric repulsion with the incoming nucleophile. If two of the hydrogens were replaced by R groups, there would be an even greater increase in steric repulsion with the incoming nucleophile.
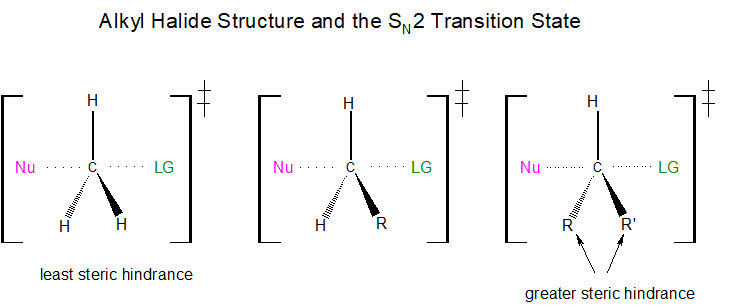
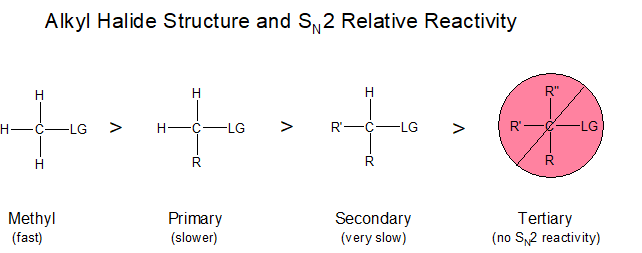
How does steric hindrance affect the rate at which an SN2 reaction will occur? As each hydrogen is replaced by an R group, the rate of reaction is significantly diminished. This is because the addition of one or two R groups shields the backside of the electrophilic carbon impeding nucleophilic penetration.
The diagram below illustrates this concept, showing that electrophilic carbons attached to three hydrogen atoms results in faster nucleophilic substitution reactions, in comparison to primary and secondary haloalkanes, which result in nucleophilic substitution reactions that occur at slower or much slower rates, respectively. Notice that a tertiary haloalkane, that which has three R groups attached, does not undergo nucleophilic substitution reactions at all. The addition of a third R group to this molecule creates a carbon that is entirely blocked.
Substitutes on Neighboring Carbons Slow Nucleophilic Substitution Reactions
Previously we learned that adding R groups to the electrophilic carbon results in nucleophilic substitution reactions that occur at a slower rate. What if R groups are added to neighboring carbons? It turns out that the addition of substitutes on neighboring carbons will slow nucleophilic substitution reactions as well.
In the example below, 2-methyl-1-bromopropane differs from 1-bromopropane in that it has a methyl group attached to the carbon that neighbors the electrophilic carbon. The addition of this methyl group results in a significant decrease in the rate of a nucleophilic substitution reaction.

If R groups were added to carbons farther away from the electrophilic carbon, we would still see a decrease in the reaction rate. However, branching at carbons farther away from the electrophilic carbon would have a much smaller effect.
Strength of the Nucleophile (Nucleophilicity)
In the SN2 reaction, the rate determining step for the reaction is the attack of the nucleophileto the substrate. Therefore, SN2 is easier to perform for stronger nucleophiles. There are predictable periodic trends in nucleophilicity. More electronegative elements hold their electrons more tightly, and are less able to donate them to form a new bond. For example, thiols (R-SH) are more nucleophilic than alcohols (R-OH) because oxygen has a higher electronegativity than sulfur. Also, negatively charged species are more nucleophilic than neutral molecules. For example methoxide anion CH3O- is more nucleophilic than methanol CH3OH. Table 4.5.1 shows a list of common nucleophiles and their relative nucleophilicity.
Table 4.5.1 Some common nucleophiles and their relative nucleophilicity. |
|
|
Weak Nucleophiles |
Strong nucleophiles |
|
H2O water R-OH alcohols R-SH thiols NH3 ammonia R-NH2 amines R-COOH carboxylic acids R-CONH2 amides |
HO ¯ hydroxide R-O¯ alkoxide R-S¯ thiolate NH2¯ Azanide R-NH¯ R-COO¯ carboxylate CN¯ cyanide N3¯ azide R¯ carbanions X¯ halides |
Resonance effects on nucleophilicity
Resonance effects also come into play when comparing the inherent nucleophilicity of different molecules. The reasoning involved is the same as that which we used to understand resonance effects on basicity. If the electron lone pair on a heteroatom is delocalized by resonance, it is inherently less reactive - meaning less nucleophilic, and also less basic. An alkoxide ion, for example, is more nucleophilic and more basic than a carboxylate group, even though in both cases the nucleophilic atom is a negatively charged oxygen. In the alkoxide, the negative charge is localized on a single oxygen, while in the carboxylate the charge is delocalized over two oxygen atoms by resonance.
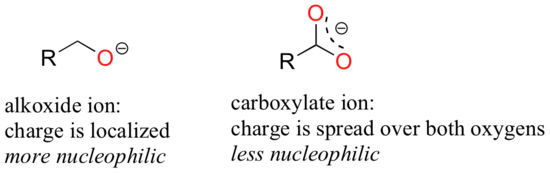
The nitrogen atom on an amide is less nucleophilic than the nitrogen of an amine, due to the resonance stabilization of the nitrogen lone pair provided by the amide carbonyl group.

The leaving group
The more stable the leaving group, the lower the transition state energy, the lower the activation energy, the faster the reaction rate. Evaluating leaving group stability is analogous to determining relative acidity by evaluating conjugate base stability. The considerations are the same: identity of the atom(s) and relative position on the periodic table, resonance delocalization, and electronegativity. Orbital hybridization is rarely relevant.
As Size Increases, Basicity Decreases, Leaving Group Stability Increases:In general, if we move from the top of the periodic table to the bottom of the periodic table as shown in the diagram below, the size of an atom will increase. As size increases, basicity will decrease, meaning a species will be less likely to act as a base; that is, the species will be less likely to share its electrons.
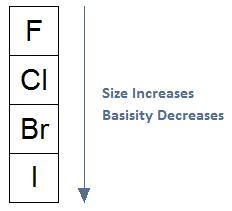
When evaluating halogens as leaving groups, the same trend is significant. Fluoride has the highest electron density and is considered the worst leaving group to the point of no reactivity. As move down the column, the leaving groups have lower electron density and greater stability with iodide considered an excellent leaving group.

slower reaction and requires a catalyst to overcome the alkoxides as poor leaving groups. The details of these two reactions will be studied in greater detail later in this text.
Solvent Effects on an SN2 reaction
The rate of an SN2 reaction is significantly influenced by the solvent in which the reaction takes place. The use of protic solvents (those, such as water or alcohols, with hydrogen-bond donating capability) decreases the power of the nucleophile through strong solvation. WE can view the nucleophile as being locked in a solvent cage through the strong hydrogen-bond interactions between solvent protons and the reactive lone pairs on the nucleophile. A less powerful nucleophile in turn means a slower SN2 reaction.
SN2 reactions are faster in polar, aprotic solvents: those that lack hydrogen-bond donating capability. Below are several polar aprotic solvents that are commonly used in the laboratory:
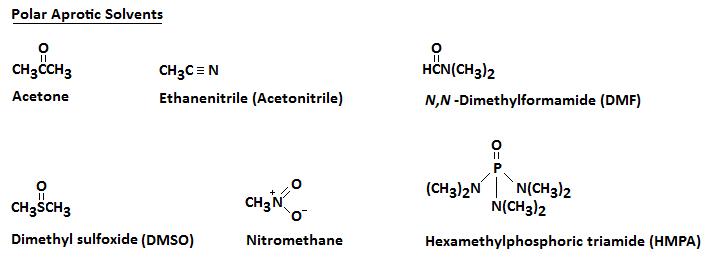
These aprotic solvents are polar but, because they do not form hydrogen bonds with the anionic nucleophile, there is a relatively weak interaction between the aprotic solvent and the nucleophile. By using an aprotic solvent we can raise the reactivity of the nucleophile.
Example
In each pair (A and B) below, which electrophile would be expected to react more rapidly in an SN2 reaction with the thiol group of cysteine as the common nucleophile?
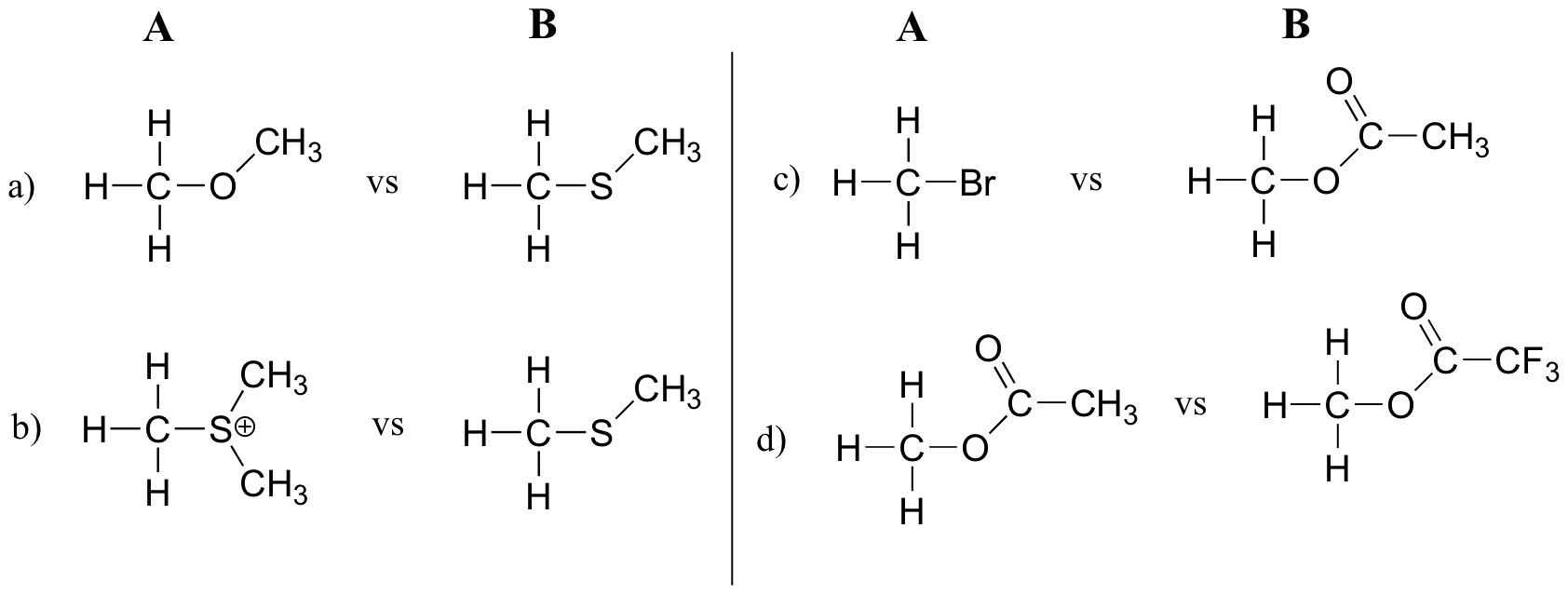
Explanations to explain differences in chemical reactivity need to discuss structural and/or electrostatic differences between the reactants
a) Cpd B b/c it has a more stable leaving group.b) Cpd A b/c it has a more stable leaving group.
c) Cpd B b/c the leaving group is resonance stabilized delocalizing the negative charge over two oxygen atoms. d) Cpd B b/c the leaving group has inductive electron withdrawal stabilization from the three fluorine atoms in addition to the resonance stabilzation.
Exercise
1. What product(s) do you expect from the reaction of 1-bromopentane with each of the following reagents in an SN2 reaction?
a) KI
b) NaOH
c) CH3C≡C-Li
d) NH3
2. Which in the following pairs is a better nuceophile?
a) (CH3CH2)2N- or (CH3CH2)2NH
b) (CH3CH2)3N or (CH3CH2)3B
c) H2O or H2S
3. Order the following in increasing reactivity for an SN2 reaction.
CH3CH2Br CH3CH2OTos (CH3CH2)3CCl (CH3CH2)2CHCl
4. Solvents benzene, ether, chloroform are non-polar and not strongly polar solvents. What effects do these solvents have on an SN2 reaction?
- Answer
-
1. (a) - (d)
2.
a) (CH3CH2)2N- as there is a charge present on the nitrogen.
b) (CH3CH2)3N because a lone pair of electrons is present.
c) H2O as oxygen is more electronegative.
3.
4. They will decrease the reactivity of the reaction.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Jim Clark (Chemguide.co.uk)
- Characteristics of the Sₙ2 Reaction. (2020, May 30). Retrieved May 23, 2021, from https://chem.libretexts.org/@go/page/45174

