2.1: Carboxylic Acids - Structures and Nomenclature
- Page ID
- 270981
Learning Objectives
- Name carboxylic acids with common names.
- Name carboxylic acids according to IUPAC nomenclature.
The characteristic functional group of carboxylic acids is the carboxyl group. This group consists of a carbonyl group attached to an OH. This OH is responsible for the acidity of this type of compounds:
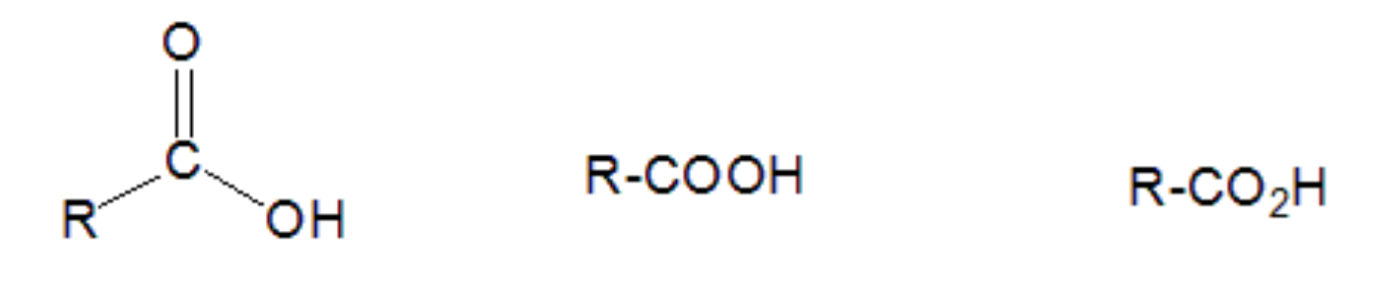
Different representations of the general formula for carboxylic acids. "R" represents the rest of the molecule.
Carboxylic acids occur widely in nature, often combined with alcohols or other functional groups, as in fats, oils, and waxes. They are components of many foods, medicines, and household products (Figure \(\PageIndex{1}\)). Not surprisingly, many of them are best known by common names based on Latin and Greek words that describe their source.

The simplest carboxylic acid, formic acid (HCOOH), was first obtained by the distillation of ants (Latin formica, meaning “ant”). The bites of some ants inject formic acid, and the stings of wasps and bees contain formic acid (as well as other poisonous materials).

The next higher homolog is acetic acid, which is made by fermenting cider and honey in the presence of oxygen. This fermentation produces vinegar, a solution containing 4%–10% acetic acid, plus a number of other compounds that add to its flavor. Acetic acid is probably the most familiar weak acid used in educational and industrial chemistry laboratories.
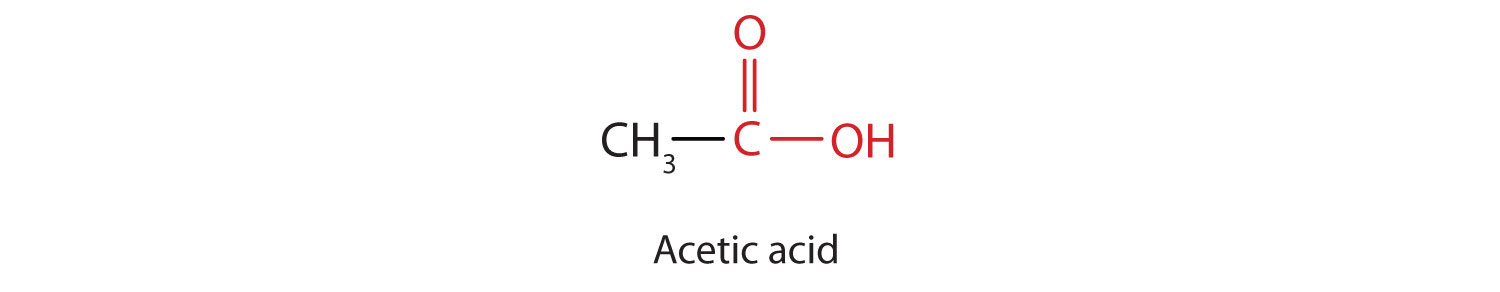
Pure acetic acid solidifies at 16.6°C, only slightly below normal room temperature. In the poorly heated laboratories of the late 19th and early 20th centuries in northern North America and Europe, acetic acid often “froze” on the storage shelf. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day.
The third homolog, propionic acid (CH3CH2COOH), is seldom encountered in everyday life. The fourth homolog, butyric acid (CH3CH2CH2COOH), is one of the most foul-smelling substances imaginable. It is found in rancid butter and is one of the ingredients of body odor. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives. Models of the first four carboxylic acids are shown in Figure \(\PageIndex{2}\).
The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid (C6H5COOH).

Common names
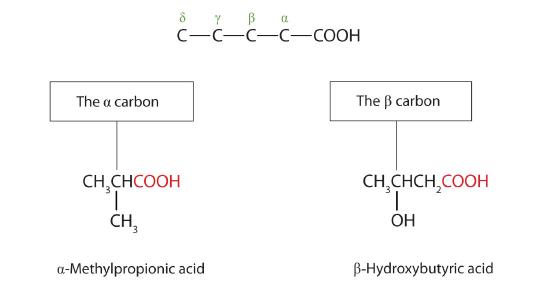
The common names of carboxylic acids use the same prefixes as for the case of aldehydes, but uses Greek letters (α, β, γ, δ, and so forth), not numbers, to designate the position of substituent groups. These letters refer to the position of the carbon atom in relation to the carboxyl carbon atom.
IUPAC names
In the nomenclature system of the International Union of Pure and Applied Chemistry (IUPAC), the parent hydrocarbon is the one that corresponds to the longest continuous chain (LCC) containing the carboxyl group. The -e ending of the parent alkane is replaced by the suffix -oic and the word acid. For example, the carboxylic acid derived from pentane is pentanoic acid (CH3CH2CH2CH2COOH). As with aldehydes, the carboxyl carbon atom is counted first; numbers are used to indicate any substituted carbon atoms in the parent chain. The position of substituents is indicated by numbers.
Greek letters are used with common names; numbers are used with IUPAC names.
Example \(\PageIndex{1}\)
Give the common and IUPAC names for each compound.
- ClCH2CH2CH2COOH
-
Solution
- The LCC contains four carbon atoms; the compound is therefore named as a substituted butyric (or butanoic) acid.
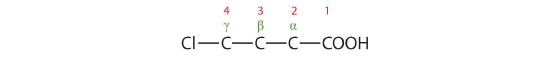
The chlorine atom is attached to the γ-carbon in the common system or C4 in the IUPAC system. The compound is γ-chlorobutyric acid or 4-chlorobutanoic acid.
- The LCC contains four carbon atoms; the compound is therefore named as a substituted butyric (or butanoic) acid.
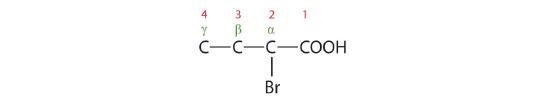
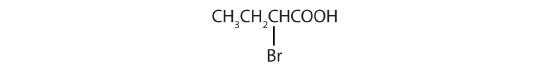
The bromine (Br) atom is at the α-carbon in the common system or C2 in the IUPAC system. The compound is α-bromobutyric acid or 2-bromobutanoic acid.
Exercise \(\PageIndex{1}\)
Give the IUPAC name for each compound.
- ClCH2CH2CH2CH2COOH
- (CH3)2CHCH2CHBrCOOH
Example \(\PageIndex{2}\)
Write the condensed structural formula for β-chloropropionic acid.
Solution
Propionic acid has three carbon atoms: C–C–COOH. Attach a chlorine (Cl) atom to the parent chain at the beta carbon atom, the second one from the carboxyl group: Cl–C–C–COOH. Then add enough hydrogen atoms to give each carbon atom four bonds: ClCH2CH2COOH.
Exercise \(\PageIndex{2}\)
Write the condensed structural formula for 4-bromo-5-methylhexanoic acid.
Concept Review Exercises
- What is the IUPAC name for the straight-chain carboxylic acid with six carbon atoms?
- The straight-chain aldehyde with five carbon atoms has the common name valeraldehyde. What is the common name of the corresponding straight-chain carboxylic acid?
Answers
- hexanoic acid
- valeric acid
Key Takeaways
- Simple carboxylic acids are best known by common names based on Latin and Greek words that describe their source (e.g., formic acid, Latin formica, meaning “ant”).
- Greek letters, not numbers, designate the position of substituted acids in the common naming convention.
- IUPAC names are derived from the LCC of the parent hydrocarbon with the -e ending of the parent alkane replaced by the suffix -oic and the word acid.
Exercises
-
Draw the structure for each compound.
- heptanoic acid
- 3-methylbutanoic acid
- 2,3-dibromobenzoic acid
- β-hydroxybutyric acid
-
Draw the structure for each compound.
- o-nitrobenzoic acid
- p-chlorobenzoic acid
- 3-chloropentanoic acid
- α-chloropropionic acid
-
Name each compound with either the IUPAC name, the common name, or both.
- (CH3)2CHCH2COOH
- (CH3)3CCH(CH3)CH2COOH
- CH2OHCH2CH2COOH
-
Name each compound with its IUPAC name.
- CH3(CH2)8COOH
- (CH3)2CHCCl2CH2CH2COOH
- CH3CHOHCH(CH2CH3)CHICOOH
Answers
-
- CH3CH2CH2CH2CH2CH2COOH
-
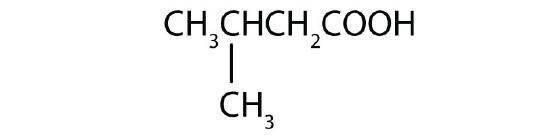
-
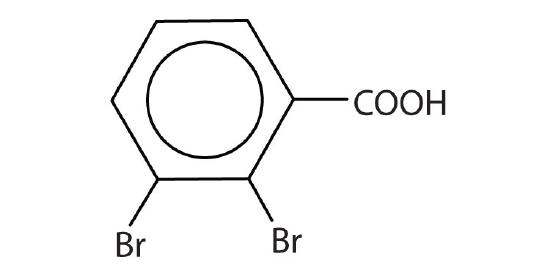
-
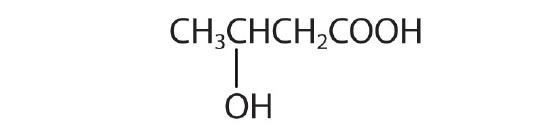
-
- 3-methylbutanoic acid; β-methylbutyric acid
- 3,4,4-trimethylpentanoic acid
- 4-hydroxybutanoic acid; γ- hydroxybutyric acid

