6.6: Phenols
- Page ID
- 221813
Learning Objectives
- To describe the structure and uses of some phenols
Structure of phenols and nomenclature
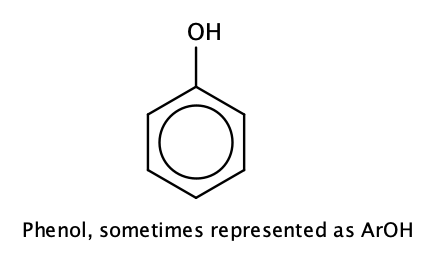
Compounds in which an OH group is attached directly to an aromatic ring are designated ArOH and called phenols.
Phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (NaOH) to form salts.
\[\ce{ArOH (aq) + NaOH (aq) \rightarrow ArONa (aq) + H_2O}\]
The parent compound, C6H5OH, is itself called phenol. (An old name, emphasizing its slight acidity, was carbolic acid.) Phenol is a white crystalline compound that has a distinctive (“hospital smell”) odor.


Physical properties of phenols
It is useful to compare phenol's melting and boiling points with those of methylbenzene (toluene). Both molecules contain the same number of electrons and are very similar shape. That means that the intermolecular attractions due to van der Waals dispersion forces are going to be very similar.
| melting point (°C) | boiling point (°C) | |
|---|---|---|
| C6H5OH | 40 - 43 | 182 |
| C6H5CH3 | -95.0 | 111 |
The reason for the higher values for phenol is in part due to permanent dipole-dipole attractions due to the electronegativity of the oxygen - but is mainly due to hydrogen bonding. Hydrogen bonds can form between a lone pair on an oxygen on one molecule and the hydrogen on the -OH group of one of its neighbors.
Chemical and biological properties of phenols
Acidity
Phenols differ from alcohols in that they are slightly acidic in water:
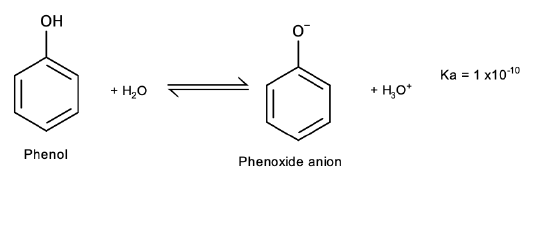
Salt formation
Also different from alcohols, phenols react with aqueous sodium hydroxide (NaOH) to form salts:
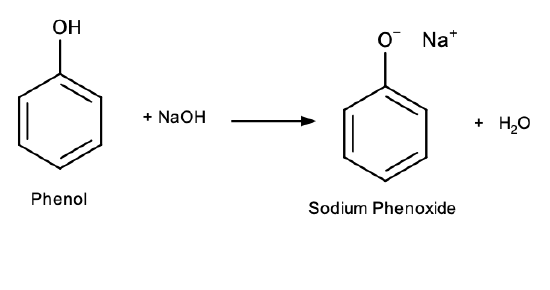
Phenol salts are ionic compounds, and therefore more soluble in water than phenol itself due to ion-dipole interactions.
Antiseptic properties
Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol. Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
An operation in 1753, painted by Gaspare Traversi, of a surgery before antiseptics were used.
One safer phenolic antiseptic is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene; resorcinol is the common name for 1,3-dihydroxybenzene, and 4-hexylresorcinol has a hexyl group on the fourth carbon atom of the resorcinol ring). It is much more powerful than phenol as a germicide and has fewer undesirable side effects. Indeed, it is safe enough to be used as the active ingredient in some mouthwashes and throat lozenges.
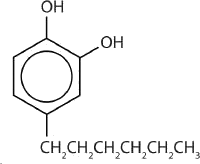
The compound 4-hexylresorcinol is mild enough to be used as the active ingredient in antiseptic preparations for use on the skin.
Antioxidant properties
Phenols act as antioxidants preventing oxidizing reactions on other compounds. Phenols such as BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) (2-tert-butyl-4-methylphenol) are widely used as antioxidants in foods, packing materials, cosmetic, and other chemical products.

Butylated hydroxyanisole (BHA) is an antioxidant consisting of a mixture of two isomeric organic compounds, 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. Image by Darkness3560, CC0, via Wikimedia Commons
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene. Image by User:Bryan Derksen, Public domain, via Wikimedia Commons
The ability of phenols to act as antioxidants is due to their ability to scavenge free radicals (R•) generated in spoiled food:
R• + PhOH → R-H + PhO•
Phenoxyl radicals (PO•) generated according to this reaction may be stabilized through resonance thus terminating the traditional chain reaction involving free radicals.
Summary
Phenols are compounds in which an OH group is attached directly to an aromatic ring. Many phenols are used as antiseptics.
Concept Review Exercises
-
How do phenols differ from alcohols in terms of structure and properties?
-
How do phenols differ in properties from aromatic hydrocarbons?
Answers
-
Phenols have an OH group attached directly to an aromatic ring. Phenols are weakly acidic.
-
Phenols have an OH group and are somewhat soluble in water.
Exercises
-
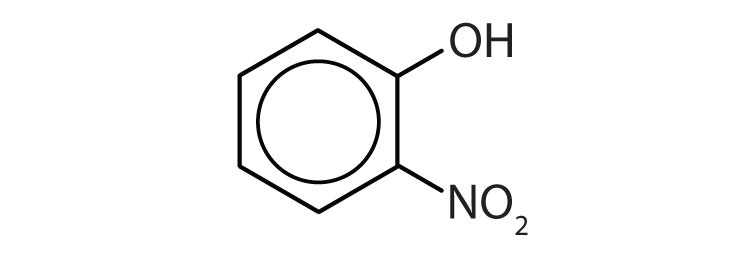
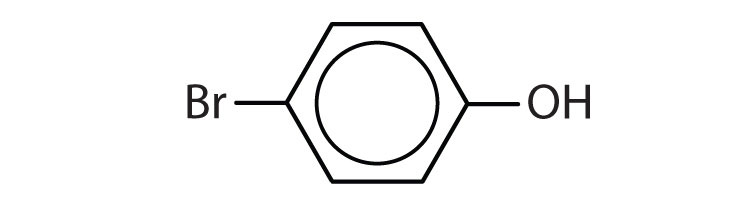
Name each compound.
-
-
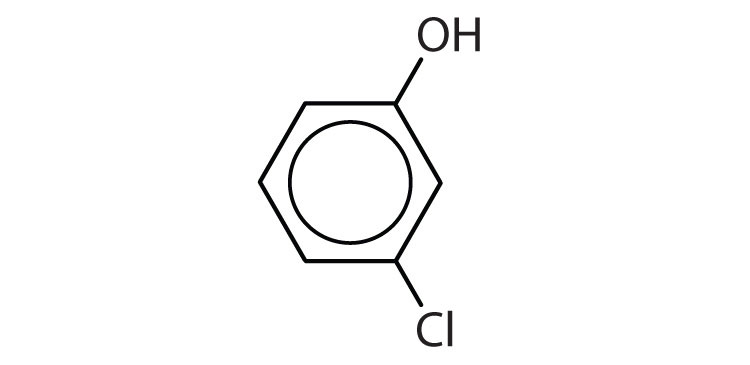
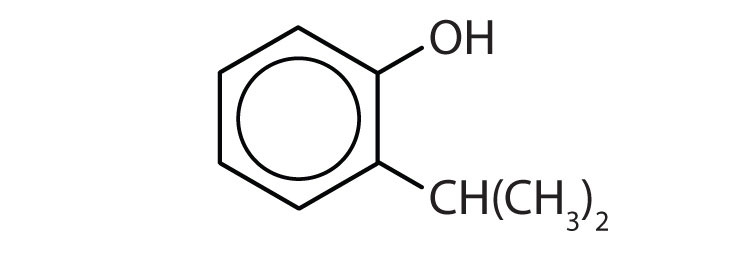
Name each compound.
-
-
Draw the structure for each compound.
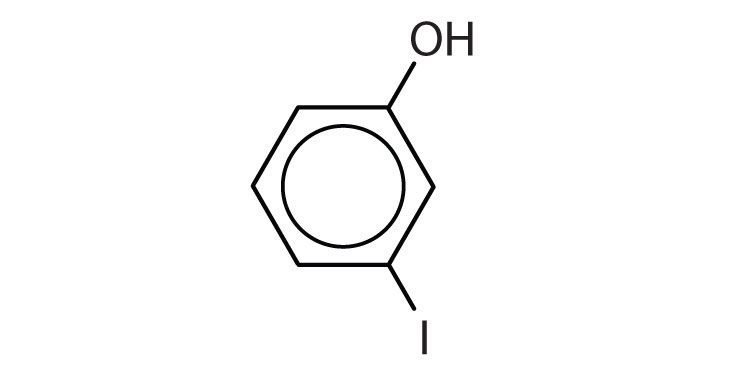
- m-iodophenol
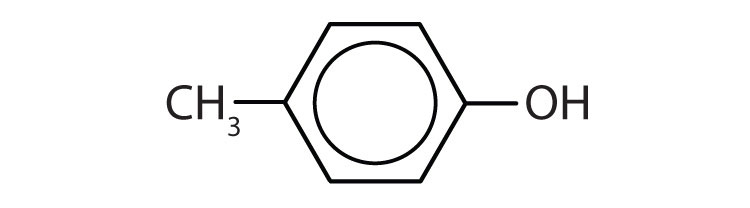
- p-methylphenol (p-cresol)
-
Draw the structure for each compound.
- 2,4,6-trinitrophenol (picric acid)
- 3,5-diethylphenol
Answers
-
- o-nitrophenol
- p-bromophenol
-
Citations and attributions
- " Phenols" by LibreTexts is licensed under CC BY-NC-SA .
- "Physical Properties of Phenol" by Jim Clark. Retrieved May 20, 2021, from https://chem.libretexts.org/@go/page/3986
- Wikipedia contributors. (2021, March 9). Butylated hydroxyanisole. In Wikipedia, The Free Encyclopedia. Retrieved 22:39, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Butylated_hydroxyanisole&oldid=1011151288
- Wikipedia contributors. (2021, April 14). Butylated hydroxytoluene. In Wikipedia, The Free Encyclopedia. Retrieved 22:39, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Butylated_hydroxytoluene&oldid=1017824735
- Wikipedia contributors. (2021, January 27). Antioxidant effect of polyphenols and natural phenols. In Wikipedia, The Free Encyclopedia. Retrieved 22:39, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Antioxidant_effect_of_polyphenols_and_natural_phenols&oldid=1003094201
- Wikipedia contributors. (2021, May 2). Phenol. In Wikipedia, The Free Encyclopedia. Retrieved 22:40, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Phenol&oldid=1021048584
-