1.6: Batteries- Using Chemistry to Generate Electricity
- Page ID
- 221440
Learning Objectives
- Identify the anode and the cathode given a diagram of an electrochemical cell
- Describe how batteries can produce electrical energy.
Electricity is an important form of energy that you use every day. It runs your calculators, cell phones, dishwashers, and watches. This form of energy involves moving electrons through a wire and using the energy of these electrons. Electrochemical cells used for power generation are called batteries. Although batteries come in many different shapes and sizes, there are a few basic types. You won't be required to remember details of the batteries, but some general information and features of each type are presented here. Many important chemical reactions involve the exchange of one or more electrons, and we can use this movement of electrons as electricity; batteries are one way of producing this type of energy. The reactions that drive electricity are called oxidation-reduction (or "redox") reactions.
Batteries
Batteries are devices that use chemical reactions to produce electrical energy. These reactions occur because the products contain less potential energy in their bonds than the reactants. The energy produced from excess potential energy not only allows the reaction to occur, but also often gives off energy to the surroundings. Some of these reactions can be physically arranged so that the energy given off is in the form of an electric current. These are the type of reactions that occur inside batteries. When a reaction is arranged to produce an electric current as it runs, the arrangement is called an electrochemical cell, a Voltaic Cell, or a Galvanic Cell.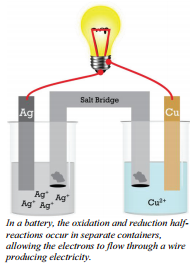
If a strip of copper is placed in a solution of silver nitrate, the following reaction takes place:
\[2 \ce{Ag^+} \left( aq \right) + \ce{Cu} \left( s \right) \rightarrow 2 \ce{Ag} \left( s\right) + \ce{Cu^{2+}} \left( aq \right)\]
In this reaction, copper atoms are donating electrons to silver ions, so the silver ions are reduced to silver atoms and copper atoms are oxidized to copper (II) ions.
As the reaction occurs, an observer would see the solution slowly turn blue (\(\ce{Cu^{2+}}\) ions are blue in solution) and a mass of solid silver atoms would build up on the copper strip.
The reaction we just described is not set up in such a way as to produce electricity. It is true that electrons are being transferred, but to produce electricity, we need electrons flowing through a wire so that we can use the energy of these electrons. This reaction, \(2 \ce{Ag^+} \left( aq \right) + \ce{Cu} \left( s \right) \rightarrow 2 \ce{Ag} \left( s \right) + \ce{Cu^{2+}} \left( aq \right)\), is one that could be arranged to produce electricity. To do this, the two half-reactions (oxidation and reduction) must occur in separate compartments, and the separate compartments must remain in contact through a salt bridge ( an ionic solution) and an external wire.
In this electrochemical cell, the copper metal must be separated from the silver ions to avoid a direct reaction. Each electrode in its solution could be represented by a half-reaction.
\[\ce{Cu} \rightarrow \ce{Cu^{2+}} + 2 \ce{e^-}\]
\[2 \ce{Ag^+} + 2 \ce{e^-} \rightarrow 2 \ce{Ag}\]
The wire connects the two halves of the reaction, allowing electrons to flow from one metal strip to the other. In this particular example, electrons will flow from the copper electrode (which is losing electrons) into the silver electrode (which is where the silver ions gain the electrons). The cell produces electricity through the wire and will continue to do so as long as there are sufficient reactants (\(\ce{Ag^+}\) and \(\ce{Cu}\)) to continue the reaction.
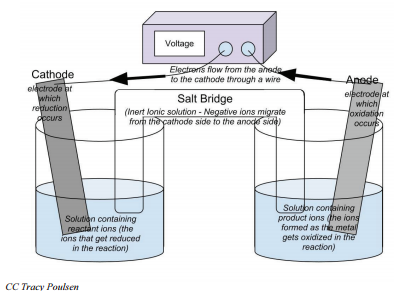
Electrochemical cells will always have two electrodes—the pieces of metal where electrons are gained or lost. (In this example, the strip of \(\ce{Ag}\) metal and \(\ce{Cu}\) metal are the electrodes.) The electrode where reduction occurs and electrons are gained is called the cathode. The electrode where oxidation occurs and electrons are lost is called the anode. Electrons will always move from the anode to the cathode. The electrons that pass through the external circuit can do useful work such as lighting lights, running cell phones, and so forth.
If the light bulb is removed from the circuit with the electrochemical cell and replaced with a voltmeter, the voltmeter will measure the cell voltage or electromotive force (Ecell or EMF) of the combination of half-cells. The size of the voltage produced by a cell depends on the temperature, the metals used for electrodes, and the concentrations of the ions in the solutions. For this particular cell, if the concentration of solutions is 1 M and the temperature is 25ºC, the initial measured voltage is 0.460 Volts. However, as the redox reaction progresses, the voltage will decrease and it becomes 0 Volts when the reactants are totally consumed. In this case, we say that the battery is dead.
It may seem complicated to construct an electrochemical cell due to all of their complexities. Electrochemical cells are actually easy to make and sometimes even occur accidentally. If you take two coins of different denomination, push them part way through the peel of a whole lemon, and then connect the two coins with a wire, a small electric current will flow.
This type of battery is known as a wet cell battery since it involves electrolytes in solution. Wet cells were the first known type of electrochemical cell to generate electricity. However, their application is limited since wet cells are prompted to leak problems. Most modern applications of electrochemical batteries involve dry cells. In a dry cell, electrolytes are used as a paste rather than as a liquid, so there less likely to leak. Batteries may be classified as primary cells (non-rechargeable) or secondary cells (rechargeable)
Some modern type of batteries are listed below:
1. Dry Cell Batteries
- Non-rechargeable (primary battery)
- Electrolytes are present as a paste rather than as a liquid.
- General purpose battery used for flashlights, transistor radios, toys, etc.
- The basic dry cell battery consists of: zinc case as the anode (oxidation); a graphite rod as the cathode (reduction) surrounded by a moist paste of either MnO2, NH4Cl, and ZnCl2 (or, in alkaline dry cells, a KOH electrolytic paste).
- General reactions for the battery: manganese (IV) oxide-zinc cell (different batteries have different reactions—you don't need to remember any of these reactions).
|
cathode
|
2MnO2(s) + 2 NH4+(aq)+ 2e- → Mn2O3 (s) + H2O(l) + 2NH3 (aq) |
|
anode
|
Zn(s) → Zn2+(aq) + 2e- |
-
- Maximum voltage of 1.5V. By connecting several cells in a series, 90V can be achieved.
- Advantages of alkaline batteries: consistent voltage, increased capacity, longer shelf life, and reliable operation at temperatures as low as -40°C.
- Disadvantage: higher cost.
2. Storage Batteries
- Rechargeable (secondary batteries)
- An example: the lead-acid battery used in cars. The anode is a grid of lead-antimony or lead-calcium alloy packed with spongy lead; the cathode is lead (IV) oxide. The electrolyte is aqueous sulfuric acid. This battery consists of numerous small cells connected in parallels (anode to anode; cathode to cathode).
- General reaction:
|
cathode
|
PbO2(s) + 4H+(aq)+ SO42-(aq) + 2e- → PbSO4 (s) + 2H2O(l) + 2NH3 (aq) |
|
anode
|
Pb(s) + SO42-(aq) → PbSO4 (s) + 2e- |
-
- Secondary batteries are recharged by passing a current through the battery in the opposite direction. In a car battery, this occurs when the engine is running.
- Other examples include the nickel-iron alkaline battery, nickel-zinc battery, nickel-cadmium alkaline battery, silver-zinc battery, and silver-cadmium battery.
3. Fuel Cells
- Fuel cells are electrochemical cells that convert the energy of a redox combustion reaction directly into electrical energy. Fuel cells require a continuous supply of reactants and a constant removal of products.
- The cathode reactant is usually air or pure oxygen; the anode fuel is a gas such as hydrogen, methane, or propane. Carbon electrodes typically contain a catalyst. The electrolyte is typically KOH.
- General reaction:
|
cathode
|
O2(g) + 2H2O(l) + 4e- → 4OH-(aq) |
|
anode
|
2H2 (g) + 4OH-(aq) → 4H2O(l) + 4e- |
| net | 2H2 (g) + O2(g) → 2H2O(l) |
-
- Advantages: no toxic waste products (water is the only product); very efficient energy conversion (70-80% efficient).
- Disadvantage: too expensive for large-scale use.
Summary
- A reaction in which there is a transfer of electrons is said to be an oxidation-reduction reaction, or a redox reaction.
- A substance that loses electrons is said to be oxidized, and the substance that gains electrons is said to be reduced.
- Redox reactions can be used in electrochemical cells to produce electricity.
- Electrochemical cells are composed of an anode and cathode in two separate solutions. These solutions are connected by a salt bridge and a conductive wire.
- An electric current consists of a flow of charged particles.
- The electrode where oxidation occurs is called the anode, and the electrode where reduction occurs is called the cathode.
Vocabulary
- Electrochemical cell - An arrangement of electrodes and ionic solutions in which a redox reaction is used to make electricity (a battery).
- Electrolysis - A chemical reaction brought about by an electric current.
- Electroplating - A process in which electrolysis is used as a means of coating an object with a layer of metal.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
- Wikipedia contributors. (2021, May 11). Electric battery. In Wikipedia, The Free Encyclopedia. Retrieved 13:37, May 21, 2021, from https://en.wikipedia.org/w/index.php?title=Electric_battery&oldid=1022586423

