3.6: Aufbau Principle
- Page ID
- 221389
Construction of a building begins at the bottom. The foundation is laid and the building goes up step by step. Construction obviously cannot start with the roof, since there is no place to hang it. The building goes from the lowest level to the highest level in a systematic way.
Aufbau Principle
In order to create ground state electron configurations for any element, it is necessary to know the way in which the atomic sublevels are organized in order of increasing energy. In principle, the electron will fill available orbitals from the lowest energy towards the higher energy. However, as the energy shell increases (quantum number n), the number of energy subshells also increases (quantum number ml). At some point, some of the subshells overlap, and the actual energy order of orbitals is not as expected. for example, the orbital in the subshell 4s have lower energy than the orbitals in the 3d subshell. Figure \(\PageIndex{1}\) represents this phenomenon:
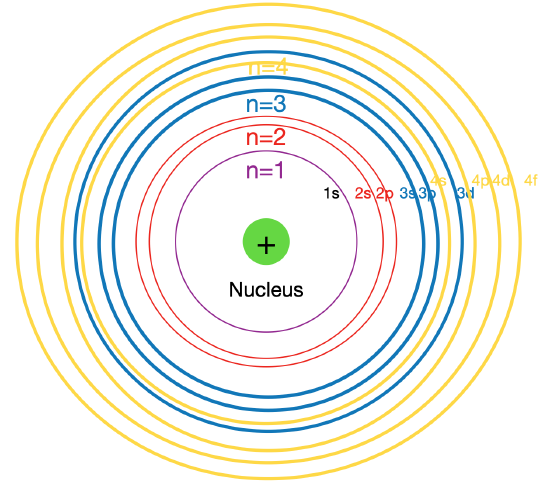
Figure \(\PageIndex{2}\):Overlap in the energy sublevels. Principle energy levels are color coded, while sublevels are grouped together, and each color presents an energy level, while each line represents an energy sublevel.
Figure \(\PageIndex{2}\) shows the order of increasing energy of the sublevels.
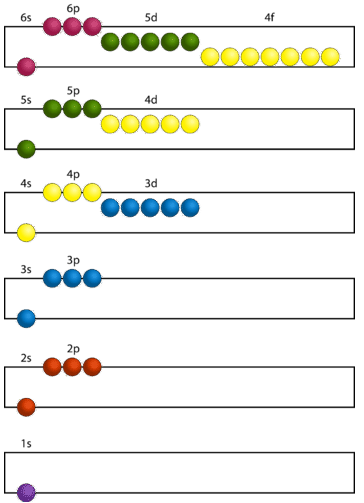
The lowest energy sublevel is always the \(1s\) sublevel, which consists of one orbital. The single electron of the hydrogen atom will occupy the \(1s\) orbital when the atom is in its ground state. As we proceed to atoms with multiple electrons, those electrons are added to the next lowest sublevel: \(2s\), \(2p\), \(3s\), and so on. The Aufbau principle states that an electron occupies orbitals in order from lowest energy to highest. The Aufbau (German for building up, construction) principle is sometimes referred to as the "building up" principle. It is worth noting that in reality, atoms are not built by adding protons and electrons one at a time, and that this method is merely an aid to understand the end result.
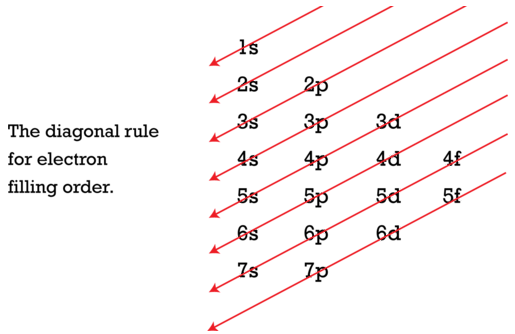
As seen in the figure above, the energies of the sublevels in different principal energy levels eventually begin to overlap. After the \(3p\) sublevel, it would seem logical that the \(3d\) sublevel should be the next lowest in energy. However, the \(4s\) sublevel is slightly lower in energy than the \(3d\) sublevel and thus fills first. Following the filling of the \(3d\) sublevel is the \(4p\), then the \(5s\) and the \(4d\). Note that the \(4f\) sublevel does not fill until just after the \(6s\) sublevel. Figure \(\PageIndex{2}\) is a useful and simple aid for keeping track of the order of fill of the atomic sublevels.
Summary
- The Aufbau principle gives the order of electron filling in an atom.
- The Aufbau principle can be used to describe the locations and energy levels of every electron in a given atom.

