Homework 2
- Page ID
- 204073
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Q1
Normalize the following molecular orbital (on two different atoms)
\[ψ = ψ_{s,A} +λψ_{s,B}\]
where \(ψ_s\) and \(ψ_{s,B}\) are normalized and \(λ\) is a parameter. Use the notation \(S\) for overlap integral to simplify the result.
Q2
The Cl−Sn−Cl bond angle in \(SnCl_2\) is close to 90°. What does this imply about the hybridization of the the central tin atom?
Q3
If the unnormalized hybrid orbitals in water that that describe bonding are expressed as
\[|\psi_A \rangle = N \left( 0.61 | 2p_z \rangle + 0.79 | 2p_x \rangle - 0.5 | 2s \rangle \right)\]
\[|\psi_B \rangle = N \left( 0.61 | 2p_z \rangle - 0.79 | 2p_x \rangle - 0.5 | 2s \rangle \right)\]
- Use these expressions calculate the \(H-O-H\) bond angle in the water molecule.
- How does this compare with the experimental values?
- How would the bond angle change if the bonding wavefunction were using non-hybridized atomic orbitals?
- Calculate the s and p characters of the two wavefunctions.
- Why isn't the \( | 2p_y \rangle\) atomic orbital also mixed into the hybrid orbitals?
Q4
What are the key aspects that separate valence bond theory from molecular orbital theory?
Q5
Demonstrate that each of the three \(sp^2\) orbitals satisfies the orthonormality criteria for wavefunctions.
Q6
Determine the valence electron configurations and bond orders in the following homonuclear diatomics: \(C^+_2\), \(C_2\), \(C^−_2\), \(N^+_2\), \(N_2\), \(N^−_2\), \(O^+_2\), \(O_2\), \(O^−_2\). Which species ones are paramagnetic?
Q7
Consider the Walsh diagram for \(AH_2\) molecules:
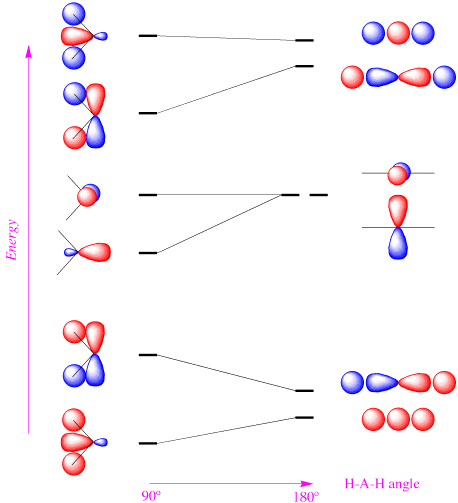
- How many valence electrons does the \(NH_2^+\) ion have?
- According to the Walsh diagram, would you expect \(NH_2^+\) to be linear or bent?
- How many valence electrons does the \(BH_2^+\) ion have?
- According to the Walsh diagram, would you expect \(BH_2^+\) to be linear or bent?

