Miscellaneous Exothermic Reactions
- Page ID
- 221963
Chemical Concept Demonstrated
- Exothermicity
Demonstrations
| A. Dichromate Volcano: |  |
| A conical pile of ammonium dichromate on an asbestos pad is ignited using a paper wick soaked in alcohol, a piece of Mg ribbon , or a flame. | |
| B. Air-Sensitive Compounds: | 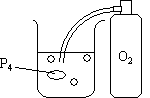 |
| A few pieces of yellow phosphorus (P4) are added to a beaker of heated water (50-70°C) and O2 is bubbled into the water. | |
| C. Gun Cotton: |  |
| Cotton is soaked in bath of H2SO4 and conc HNO3 for hours, and rinsed repeatedly with water. A baseball-size piece of gun cotton is placed on an asbestos pad, and ignited with a candle. |
D. Sugar Dehydration: Add sulfuric acid to a beaker 1/4 full of sugar.
E. Methanol-CrO3 Combustion: Half a gram of CrO3 on a watch glass is squirted with methanol.
F. Thermite Alternative: Add a few drops of glycerin to the top of a cone-shaped pile of KMnO4.
G. Smoke Screens:
-Mix 4 g I2 and 4 g Al, then add a little water.
-Mix 4 g NH4Cl, 1 g NH4NO3, and 4 g powdered Zn, then add a little water.
-Mix 2 g of zinc and 1 g of sulfur. Thrust a red-hot wire into the mixture.
H. Sulfur Oxidation: Pieces of sulfur are added to a mixture of molten KNO3 and charcoal in a test tube.
I. Sodium Peroxide Combustion: Na2O2 (in a cotton crucible) is given a few drops of water.
J. Carbide Cannon: The commercially-sold Big-BangTM cannon (made by the Conestoga Company of Bethlehem, PA) is loaded with water and its ammunition, BangsiteTM (actually just calcium carbide). The ignitor is then triggered.
K. Fire Rocks: Ice covered with calcium carbide is ignited with a dropped match.
Observations
- Dichromate Volcano: The top of the conical pile erupts in a volcanic manner.
- Air Sensitive Compounds: At every point of contact between the oxygen and the phosphorus, a fireball shoots up from the water.
- Gun Cotton: The cotton instantly disappears into a fireball as soon as it touches the flame.
- Sugar Dehydration: A black pillar of carbon slowly rises up out of the beaker, and some heat is produced.
- Methanol-CrO3 Combustion: The methanol bursts into flames.
- Thermite Alternative: A shower of sparks comes out of the conical pile.
- Smoke Screens: In all cases, clouds of thick smoke are produced.
- Sulfur Oxidation: A flame will shoot through the test tube each time a piece of sulfur is added.
- Sodium Peroxide Combustion: The Na2O2 combusts and takes the cotton crucible with it.
- Carbide Cannon: Upon ignition, the cannon produces a loud bang and a slight flash.
- Fire Rocks: The reaction proceeds as a series of pops and flashes which consume the ice and carbide.
Explanation
All of the above reactions are examples of exothermic reactions. They are exothermic because the energy state of the products is less than that of the reactants, and the difference in energy is given off as heat.
Contributors
- Dr. George Bodner (Perdue University)

