The Sb/I₂ Reaction
- Page ID
- 222039
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Chemical Concepts Demonstrated
- Introduction to chemical reactions
- Stoichiometry of reactions
- Limiting reagents
Demonstration
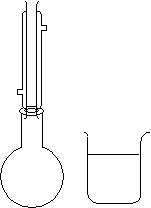
| Samples of Sb and I2 (Sb is in excess) are added to toluene inside the reflux apparatus.
The solution is filtered while still hot. After refluxing, the solution is poured into the beaker and immersed in an ice bath. The solution is then filtered after it is cooled. |
 |
Observations
After the solution has been cooled, SbI3 product is collected.
Explanation
\[\ce{2 Sb (s) + 3 I2 (s) -> 2 SbI3 (s)}\]
SbI3 is soluble in toluene at reflux temperature, but Sb is not. The excess Sb is removed by filtering the solution while the it is still hot. When SbI3 is cooled it becomes insoluable and the product can be collected by filtering the solution again.
The reaction has been used in the past to expose students to the rudiments of stoichiometry.
Contributors
- Dr. George Bodner (Perdue University)

