20.S: Carboxylic Acids and Nitriles (Summary)
- Page ID
- 329349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
20.0 Chapter Objectives and Introduction to Carboxylic Acids
- Carboxylic acids are defined as molecules that have a hydroxyl group bonded to a carbonyl group (RCOOH).
- Fatty acids are naturally occurring carboxylic acids that are important components of lipids.
- Fatty acids can either be saturated (all carbon-carbon bonds are single bonds) and unsaturated (one or more carbon-carbon double bonds).
- Several other functional groups can be prepared from carboxylic acids and are considered derivatives (acyl halides, anhydrides, esters, amides and nitriles).
20.1 Naming Carboxylic Acids and Nitriles
- Carboxylic acids are named following IUPAC rules with ending -oic acid.
- When carboxylic acids are named as an attachment to a ring, add -carboxylic acid to the name of the cyclic compound.
- Nitriles are named following IUPAC rules with the ending -nitrile or -carbonitrile.
20.2 Structure and Properties of Carboxylic Acids
- Carboxylic acids have trigonal planar shape around the carbonyl carbon and due to resonance structures, all the atoms of the functional group are co-planar.
- Conjugation in carboxylic acids increases acidity of the hydroxyl hydrogen compared to alcohols. The conjugate base (carboxylate) is stabilized by resonance.
- Carboxylic acids form hydrogen bonded dimers yielding higher boiling points than for other molecules of similar size.
- Small carboxylic acids are often soluble in water due to hydrogen bonding. Larger carboxylic acids are often slightly soluble in water, though when converted to a carboxylate salt by treating with base, become water soluble.
20.3 Biological Acids and the Henderson-Hasselbalch Equation
- Carboxylic acids are fully protonated in acidic solutions.
- Carboxylic acids are fully dissociated in basic solutions.
- The Henderson-Hasselbalch equation can be used to relate pH, pKa and ratio of concentrations of acid and conjugate base.
20.4 Substituent Effects on Acidity
- Withdrawing groups (through inductive or resonance effects) can stabilize carboxylates, increasing acidity of carboxylic acids.
- Donating groups (through inductive or resonance effects) destabilize carboxylates, decreasing acidity of carboxylic acids.
- Nearly all substituents in ortho positions increase acid strength whether donating or withdrawing, this is called the ortho-effect.
20.5 Preparing Carboxylic Acids
- Carboxylic acids can be prepared through oxidation of several functional groups including: 1o alcohols, aldehydes, alkyl arene side chains, and alkenes.
- Carboxylic acids can also be prepared by carboxylation of Grignard reagents and hydrolysis of nitriles.
20.6 Reactions of Carboxylic Acids: An Overview
- There are four main categories of carboxylic acid reactions:
- Acid/base reaction (salt formation)
- Nucleophilic acyl substitution
- Reduction
- Substitution alpha to the carbonyl
- Nitriles have linear geometry due to the sp hybrid orbitals on carbon and nitrogen.
- Nitriles are less basic than amines.
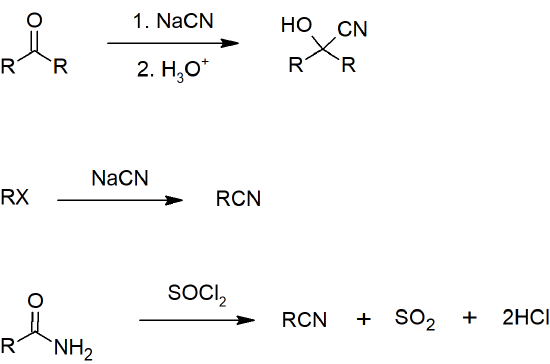
- Nitriles can be prepared from aldehydes, ketones, or amides.
- Nitriles react similarly to carbonyl compounds where nucleophiles will attack the carbon end of the nitrile.
- Nitriles can be hydrolyzed to carboxylic acids under basic or acidic conditions.
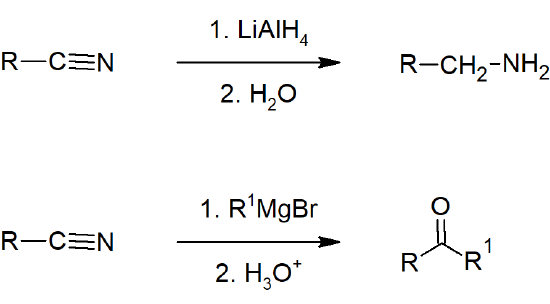
- Nitriles can be reduced to amines by hydride reduction.
- Nitriles can be converted to aldehyde by reaction with DIBALH.
- Nitriles can be converted to ketones by reaction with organometallic compounds.
20.8 Spectroscopy of Carboxylic Acids and Nitriles
- IR of carboxylic acids typically show a very strong and broad OH stretch from about 2500 to 3300 cm-1 as well as a strong carbonyl stretch around 1710 cm-1.
- 1H NMR of carboxylic acids show the OH proton between 10-12 ppm as well as hydrogens on carbon adjacent to the carbonyl around 2-3 ppm.
- 13C NMR of carboxylic acids show the carbonyl carbon at 160-180 ppm.
- Mass spectra of carboxylic acids often have fragments for OH (loss of 17) and COOH (loss of 45).
- The most identifying characteristic of IR of nitriles is the CN triple bond stretch which appears near 2250 cm-1.
- 1H NMR of nitriles show protons on the carbon next to the nitrile at around 2-3 ppm.
- 13C NMR of nitriles show the nitrile carbon in the 115-120 ppm range.
- Mass spectra of nitriles will often not have a visible molecular ion, but instead exhibit a strong M-1 peak.
Skills to Master
- Skill 20.1 Name carboxylic acids and nitriles using IUPAC rules.
- Skill 20.2 Draw the structure of carboxylic acids and nitriles from the IUPAC name.
- Skill 20.3 Describe the geometries and approximate bond angles of carboxylic acids and nitriles.
- Skill 20.4 Explain the acidity of carboxylic acids based on conjugate base stabilization.
- Skill 20.5 Explain physical properties of carboxylic acids.
- Skill 20.6 Describe the structure of carboxylic acids in buffered solutions.
- Skill 20.7 Use the Henderson-Hasselbach equation to relate pH, pKa, and acid/conjugate base concentrations.
- Skill 20.8 Explain inductive effects on acidity.
- Skill 20.9 Explain relative acidity of aromatic acids.
- Skill 20.10 Draw mechanisms for preparing carboxylic acids including:
- Oxidations
- Carboxylation of Grignard reagents
- Hydrolysis of Nitriles
- Skill 20.11 List the four categories of carboxylic acid reactions.
- Skill 20.12 Draw reactions for preparing nitriles including formation from:
- aldehyde or ketone
- 1o amide
- Skill 20.13 List general reactions of nitriles.
- Skill 20.14 Draw mechanisms for reactions of nitriles including:
- Hydrolysis to acids (acidic and basic mechanisms)
- Reduction to 1o amines
- Conversion to aldehyde by reaction with DIBALH.
- Conversion to ketones by reaction with organometallic compounds.
- Skill 20.15 Plan a synthesis of a ketone from a nitrile.
- Skill 20.16 Use IR, NMR and MS to identify carboxylic acids and nitriles.
Summary of Reactions
Carboxylic Acid Preparation
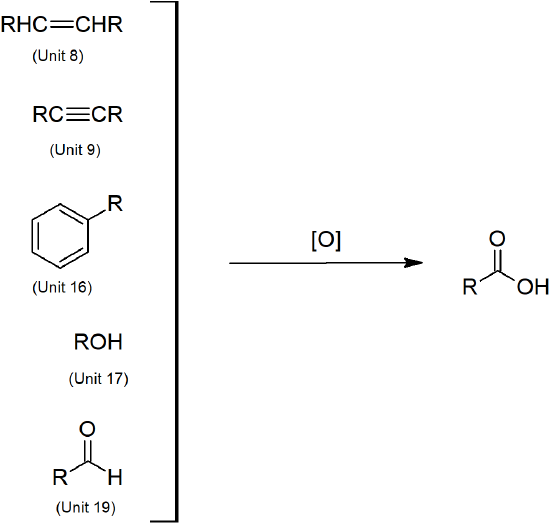
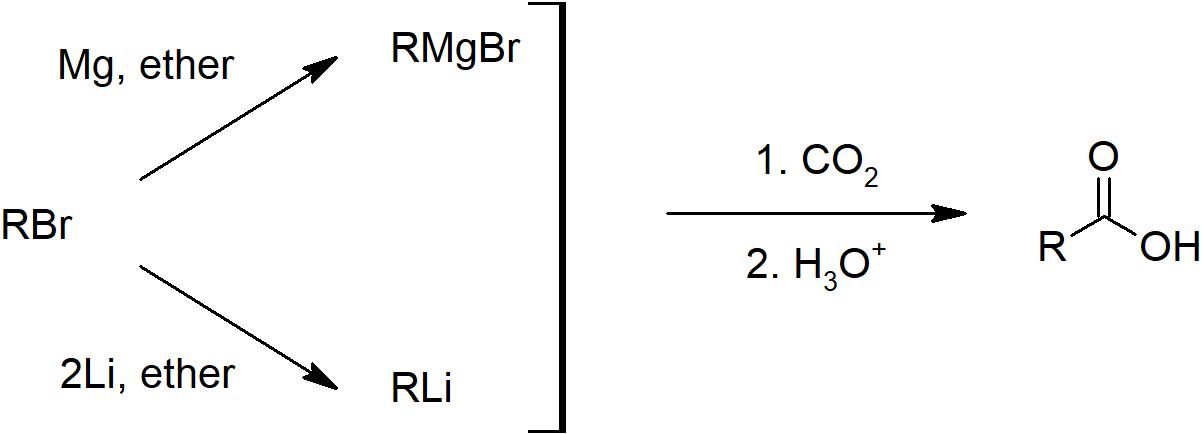

Nitrile Preparation
Reaction of Nitriles
Contributor
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Layne Morsch (University of Illinois Springfield)

