20.7 Chemistry of Nitriles
- Page ID
- 90987
Objectives
After completing this section, you should be able to
- discuss, in detail, the preparation of nitriles:
- write an equation to illustrate the formation of a nitrile by the nucleophilic attack of cyanide ion on an alkyl halide.
- write an equation to illustrate the formation of a nitrile by the dehydration of a primary amide.
- identify the product formed when a primary amide is treated with SOCl2, P2O5, or POCl3.
- identify the primary amide, the reagents, or both, needed to prepare a given nitrile by a dehydration reaction.
- write a detailed mechanism for the dehydration of a primary amide by thionyl chloride.
- discuss, in detail, the reactions of nitriles:
- write an equation to describe the (acidic or basic) hydrolysis of a nitrile.
- write detailed mechanisms for the acidic and basic hydrolysis of nitriles.
- identify the products formed from the (acidic or basic) hydrolysis of a given nitrile.
- identify the nitrile, the reagents, or both, needed to obtain a given carboxylic acid from a hydrolysis reaction.
- write an equation to describe the reduction of a nitrile to give a primary amine.
- identify the product formed from the lithium aluminum hydride reduction of a given nitrile.
- identify the nitrile, the reagents, or both, needed to prepare a given amine by direct reduction.
- write a detailed mechanism for the reduction of a nitrile to a primary amine using lithium aluminum hydride.
- give an example of the reduction of a nitrile with diisobutylaluminum hydride.
- write an equation to illustrate the reaction of a nitrile with a Grignard reagent.
- identify the product formed from the reaction of a given nitrile with a given Grignard reagent.
- identify the nitrile, the Grignard reagent, or both, needed to prepare a given ketone.
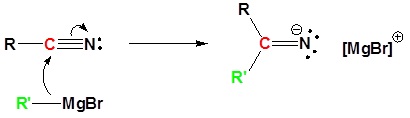
- write a detailed mechanism for the reaction of a nitrile with a Grignard reagent.
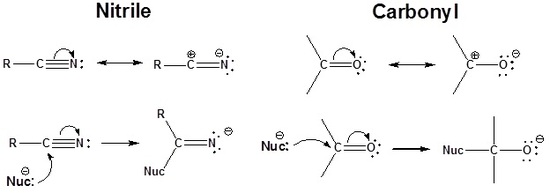
To be able to understand the driving force behind the reactions of nitriles, you must recognize the polarity of this group:
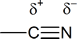
You can therefore expect to see similarities between the behaviour of the nitrile group and the similarly polarized carbonyl group:
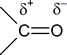
Properties of Nitriles
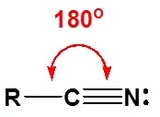
The electronic structure of nitriles is very similar to that of an alkyne with the main difference being the presence of a set of lone pair electrons on the nitrogen. Both the carbon and the nitrogen are sp hydridized which leaves them both with two p orbitals which overlap to form the two \(\pi\) bond in the triple bond. The R-C-N bond angle in and nitrile is 180o which give a nitrile functional group a linear shape.
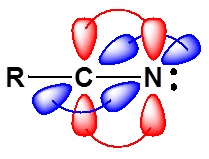
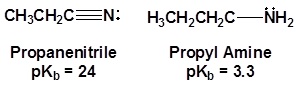
The lone pair electrons on the nitrogen are contained in a sp hybrid orbital which makes them much less basic and an amine. The 50% character of an sp hybrid orbital close to the nucleus and therefore less basic compared to other nitrogen containing compounds such as amines.
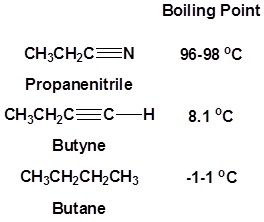
The presence of an electronegative nitrogen causes nitriles to be very polar molecules. Consequently, nitriles tend to have higher boiling points than molecules with a similar size.
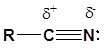
Interesting Nitriles
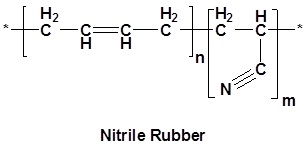
One of the most common occurrences of nitriles is in Nitrile rubber. Nitrile rubber is a synthetic copolymer of acrylonitrile and butadiene. This form of rubber is highly resistant to chemicals and is used to make protective gloves, hoses and seals.
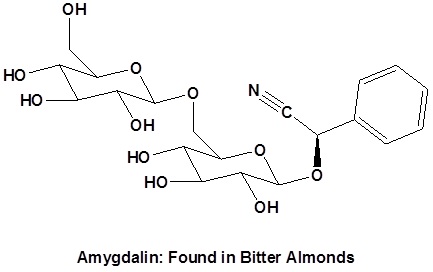
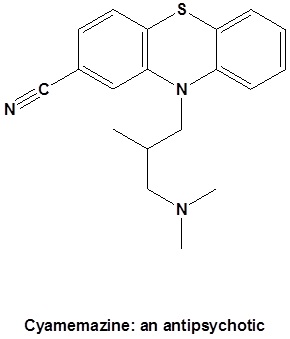
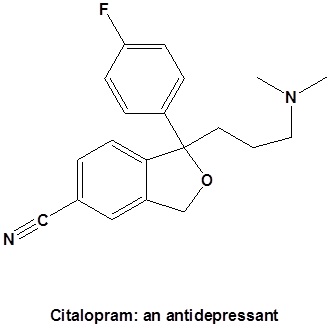
Preparation of Nitriles
Addition of cyanide (-:C≡N) to an aldehyde or ketone forms a cyanohydrin

Nitriles are formed by an SN2 reaction between a bromide and sodium cyanide

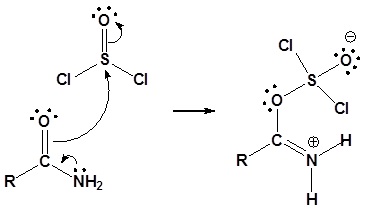
1o Amides can be converted to nitriles by dehydration with thionyl chloride (or other dehydrating agents like P2O5, or POCl3).
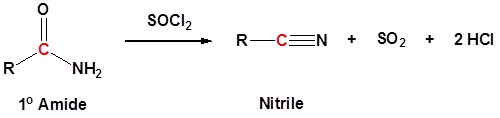
Mechanism
1) Nucleophilic attach on thionyl chloride
2) Leaving group removal
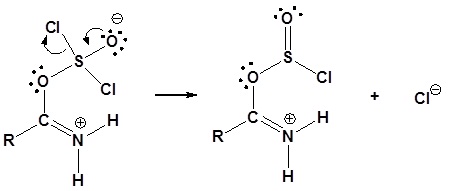
3) Deprotonation
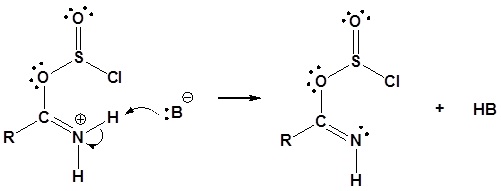
4) Leaving group removal
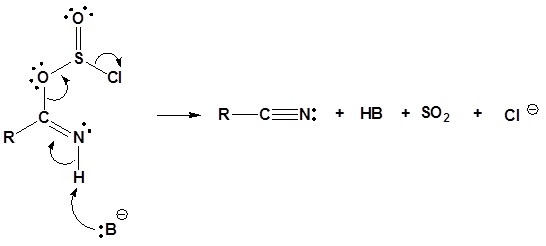
Reactions of Nitriles
The carbon in a nitrile is electrophilic because a resonance structure can be drawn which places a positive charge on it. Because of this the triple bond of a nitrile accepts a nucleophile in a manner similar to a carbonyl.
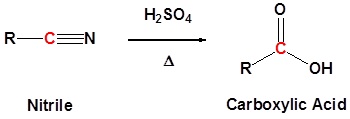
Nitriles can be converted to carboxylic acid with heating in sulfuric acid. During the reaction an amide intermediate is formed.
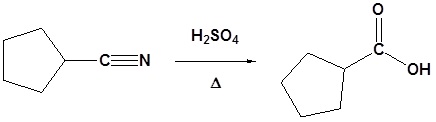
Example
Mechanism
1) Protonation

2) Nucleophilic attack by water
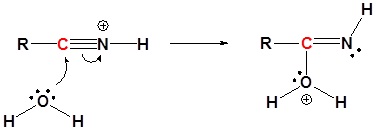
3) Proton Transfer
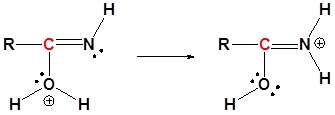
4) Resonance
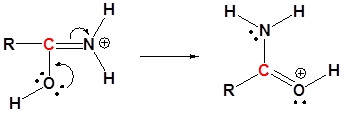
5) Deprotonation
6) Further hydrolysis of the amide. This mechanism can be found in Section 21.7
Nitriles can be converted to 1° amines by reaction with LiAlH4
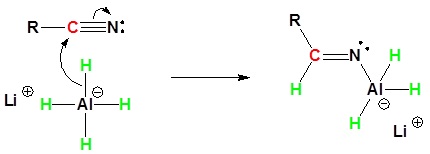
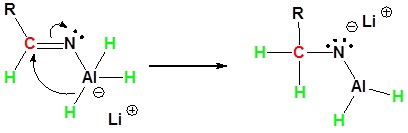
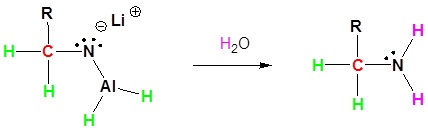
During this reaction the hydride nucleophile attacks the electrophilic carbon in the nitrile to form an imine anion. Once stabilized by a Lewis acid-base complexation the imine salt can accept a second hydride to form a dianion. The dianion can then be converted to an amine by addition of water.
General Reaction
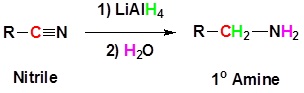
Going from reactants to products simplified
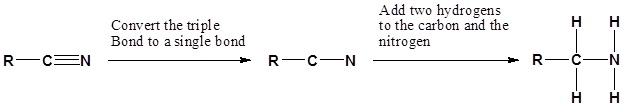
Example
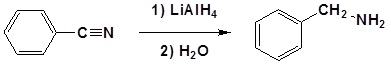
Mechanism
1) Nucleophilic Attack by the Hydride
2) Second nucleophilic attack by the hydride.
3) Protonation by addition of water to give an amine
Reaction of Nitriles with Organometaliic Reagents
Grignard reagents can attack the electophillic carbon in a nitrile to form an imine salt. This salt can then be hydrolyzed to become a ketone.
General Reaction
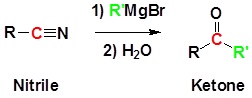
Example
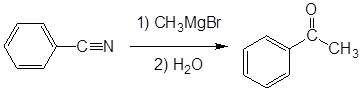
Mechanism
1) Nucleophilic Attack by the Grignard Reagent
2) Protonation
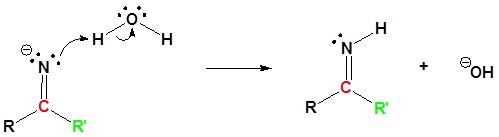
3) Protonation
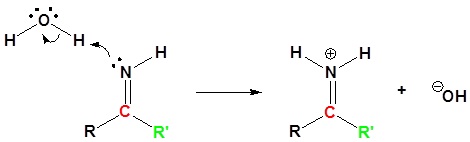
4) Nucleophilic attack by water
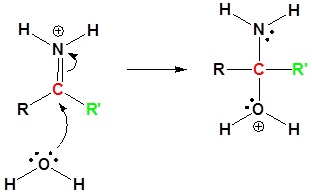
5) Proton Transfer

6) Leaving group removal
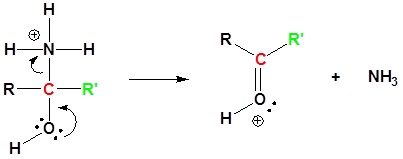
7) Deprotonation
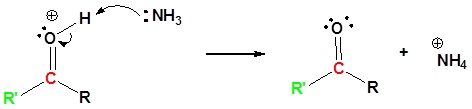
Exercises
Show how the following compounds could be prepared from a nitrile:
a)
b)
- Answer
-
a)
b)
Show how the following transformations could be preformed:
a)
b)
- Answer
-
a)
b)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

