20.4 Substituent Effects on Acidity
- Page ID
- 90984
Objectives
After completing this section, you should be able to
- list a given series of carboxylic acids in order of increasing or decreasing acidity.
- explain the difference in acidity between two or more given carboxylic acids.
- arrange a series of substituted benzoic acids in order of increasing or decreasing acidity.
- determine whether a given substituted benzoic acid will be more or less acidic than benzoic acid.
- decide which of two or more substituted benzoic acids is the most acidic, and explain your decision on the basis of the electron‑withdrawing or electron‑releasing ability of the substituent.
- use the Ka (or pKa ) of a substituted benzoic acid to predict the effect that the substituent has on the susceptibility of the benzene ring to electrophilic attack.
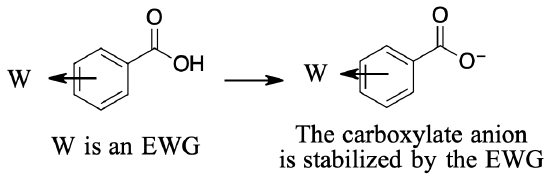
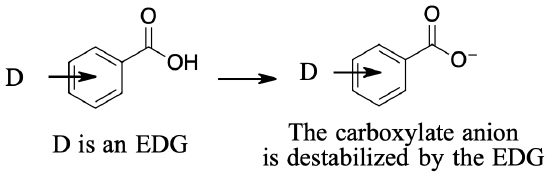
You have already seen how the presence of an electron‑withdrawing or electron‑releasing group affects the stability of a positively charged carbocation. Now you see how these groups affect the stability of carboxylate anions, and in turn, determine the dissociation constant of a carboxylic acid.
The resonance effect described here is undoubtedly the major contributor to the exceptional acidity of carboxylic acids. However, inductive effects also play a role. For example, alcohols have pKa's of 16 or greater but their acidity is increased by electron withdrawing substituents on the alkyl group. The following diagram illustrates this factor for several simple inorganic and organic compounds (row #1), and shows how inductive electron withdrawal may also increase the acidity of carboxylic acids (rows #2 & 3). The acidic hydrogen is colored red in all examples.
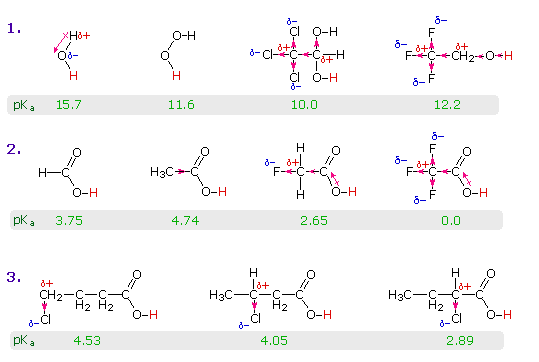
Water is less acidic than hydrogen peroxide because hydrogen is less electronegative than oxygen, and the covalent bond joining these atoms is polarized in the manner shown. Alcohols are slightly less acidic than water, due to the poor electronegativity of carbon, but chloral hydrate, Cl3CCH(OH)2, and 2,2,2,-trifluoroethanol are significantly more acidic than water, due to inductive electron withdrawal by the electronegative halogens (and the second oxygen in chloral hydrate). In the case of carboxylic acids, if the electrophilic character of the carbonyl carbon is decreased the acidity of the carboxylic acid will also decrease. Similarly, an increase in its electrophilicity will increase the acidity of the acid. Acetic acid is ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donating character of an alkyl group relative to hydrogen, as noted earlier in a discussion of carbocation stability. Electronegative substituents increase acidity by inductive electron withdrawal. As expected, the higher the electronegativity of the substituent the greater the increase in acidity (F > Cl > Br > I), and the closer the substituent is to the carboxyl group the greater is its effect (isomers in the 3rd row). Substituents also influence the acidity of benzoic acid derivatives, but resonance effects compete with inductive effects. The methoxy group is electron donating and the nitro group is electron withdrawing (last three entries in the table of pKa values).
Withdrawing Inductive Effects
A fluorine atom is more electronegative than a hydrogen atom, and thus it is able to ‘induce’, or ‘pull’ the electron density of covalent bonds towards itself. In the fluoroacetate anion, the electrons in the C-F covalent bond are pulled toward the fluorine giving the carbon a partial positive charge. The positively charged carbon, in turn, draws electron density away from the carboxylate anion, dispersing the charge, and creating a stabilizing effect. Stabilizing the carboxylate anion increases the acidity of the corresponding carboxylic acid. In this context, the fluorine substituent is acting as an electron-withdrawing group.
Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine
A similar effect is seen when other electron-withdrawing groups are attached to -CH2CO2H. As the power of the electron-withdrawing group becomes stronger there is a corresponding drop in the pKa of the carboxylic acid.
The presence of multiple electron-withdrawing groups compounds the inductive effect and continues to increase the acidity of the carboxylic acid. Dichloroacetic is a stronger acid than chloroacetic acid, and trichloroacetic acid is even stronger.
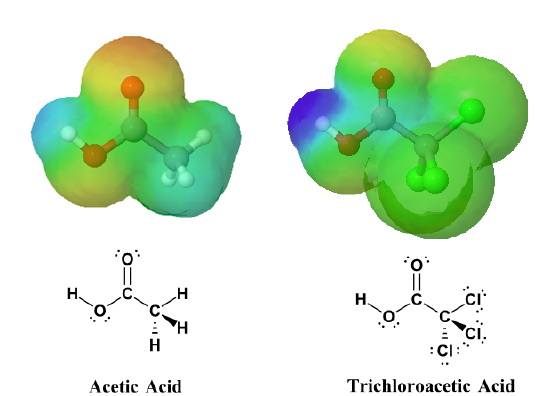
The inductive effects of chlorine be clearly seen when looking at the electrostatic potential maps of acetic acid (Left) and trichloroacetic acid (Right). The O-H bond in trichloroacetic acid is highly polarized, as shown by the dark blue color making it a much stronger acid than acetic acid.
Because inductive effects are not transmitted effectively through covalent bonds, the acid-strengthening effect falls off rapidly as the number of sigma bonds between the carboxylic acid and the electron-withdrawing group increases. A distance of three sigma bonds almost completely eliminates chlorine's inductive effect in 4-chlorobutanoic acid, giving it a similar pKa value to unsubstituted butanoic acid.
Donating Inductive Effects
Alkyl groups (hydrocarbons) are inductively electron-donating. In this case, the inductive effects pushes electron density onto the carboxylate anion, producing a destabilizing effect, decreasing the acidity of the carboxylic acid.
Lengthening the alkyl chain of a carboxylic acid can increase this inductive effect but it no longer decreases the acidity further after the chain is about three carbons long.
Acidity of Substituted Benzoic Acids
Notice the trend in the following table where electron donating substituents (X) at the para position lead to weaker acids while those having more electron withdrawing groups, further down the table, generate stronger acids.
|
X |
pKa |
|
|---|---|---|
| —N(CH3)2 | 6.03 |  |
| —NHCH3 | 5.04 | |
| —OH | 4.57 | |
| —OCH3 | 4.50 | |
| —C(CH3)3 | 4.38 | |
| —H | 4.20 | |
| —Cl | 4.00 | |
| —Br | 3.96 | |
| —CHO | 3.77 | |
| —CN | 3.55 | |
| —NO2 | 3.43 |
The following molecule, p-cyanobenzoic acid, has a pKa of 3.55. Does the cyano substituent activate or deactivate the aromatic ring towards electrophilic aromatic substitution?
Solution
The pKa of benzoic acid is 4.2 which means it is a weaker acid than p-cyanobenzoic acid. This this means that the cyano substituent is deactivating the ring.
Exercises
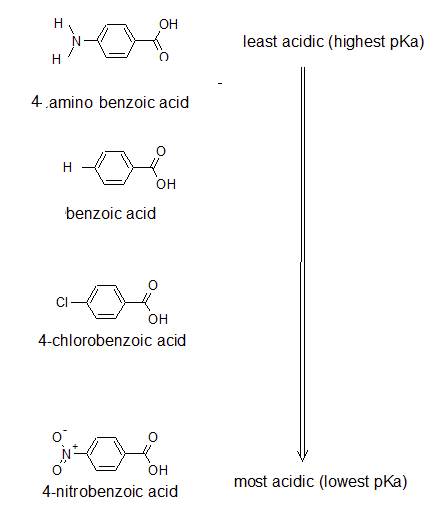
Draw the bond-line structures and arrange the following compounds in order of increasing acidity: 4-nitrobenzoic acid; 4-aminobenzoic acid; 4-chlorobenzoic acid; and benzoic acid. Try to use the expected inductive effects of the substituents to determine the acidity rather than looking at the pKa table.
- Answer
-
For the following pairs, which is expected to be the stronger acid? Explain your answer.
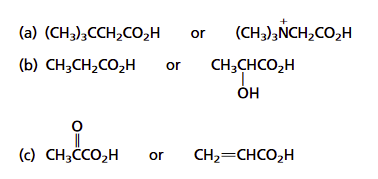
- Answer
-
a) Consider the inductive effects of the substituents attached to the carboxylic acid. The tert-butyl group is electron-donating which should decrease the acidity of the carboxylic acid. The trimethylammonium substituent is positively charged and can be a powerful electron-withdrawing substituent. This should increase the acidity of the carboxylic acid. The compound (CH3)3NCH2CO2H is expected to be a stronger acid than (CH3)3CCH2CO2H. The acidity constants for these two compounds match the predictions.
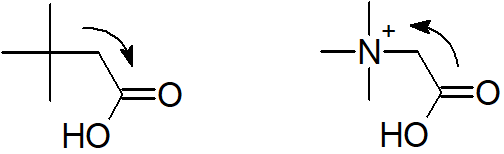
b) Having an electron-withdrawing hydroxyl group at the C-2 stabilizes the carboxylate ion of lactic acid through inductive effects.
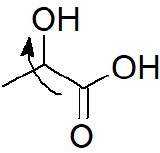
This should make lactic acid more acidic than propanoic acid.
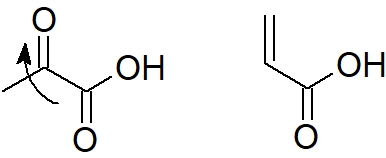
c) Due to the presence of a highly electronegative oxygen, the carbonyl group is expected to be more strongly electron-withdrawing than a carbon–carbon double bond. Thus, pyruvic acid should be a stronger acid than acrylic acid.
Oxalic acid is a dicarboxylic acid with two acidic protons. The first proton is much more acidic (pKa = 1.20) than a typical carboxylic acid. However, Heptanedioic acid's first acidic proton has a pKa much closer to that of a typical carboxylic acid. Explain these differences.
- Answer
-
With oxalic acid one carboxyl group acts as an inductive electron-withdrawing group which increases the acidity of the other carboxylic acid. This inductive effect is only relevant with the two carboxyl groups are separated by only a few bonds. In heptanedioic acid, the carboxyl groups are separated by five carbon which effectively negates the inductive effect.
The carboxylic acid of 4-formylbenzoic acid has a pKa of 3.75. Is this molecule likely to be more reactive or less reactive than benzene toward electrophilic aromatic substitution?
- Answer
-
Benzoic acid (pKa = 4.2) has a higher pKa and is more acidic than 4-formylbenzoic acid (pKa = 3.75). This means that the formyl group is removing electrons from the aromatic ring making it deactivated toward electrophilic aromatic substitution.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry