18.S: Ethers and Epoxides; Thiols and Sulfides (Summary)
- Page ID
- 207107
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
- Ethers are molecules containing oxygen which is bonded to two carbon groups.
- Thiols are sulfur analogues of alcohols with an SH group instead of OH.
- Sulfides are sulfur analogues of ethers with sulfur bonded to two carbon groups instead of oxygen.
18.1 Names and Properties of Ethers
- Ether groups are named as alkoxy groups.
- Ethers do not have intramolecular hydrogen bonding (unlike alcohols), therefore ethers have significantly reduced intermolecular forces causing boiling points that are much lower than similar sized alcohols.
- Alkoxymercuration can be used to prepare an ether from an alkene.
18.3 Reactions of Ethers: Acidic Cleavage
- The carbon-oxygen bonds of ethers can be cleaved with strong acids through either nucleophilic substitution or elimination reactions.
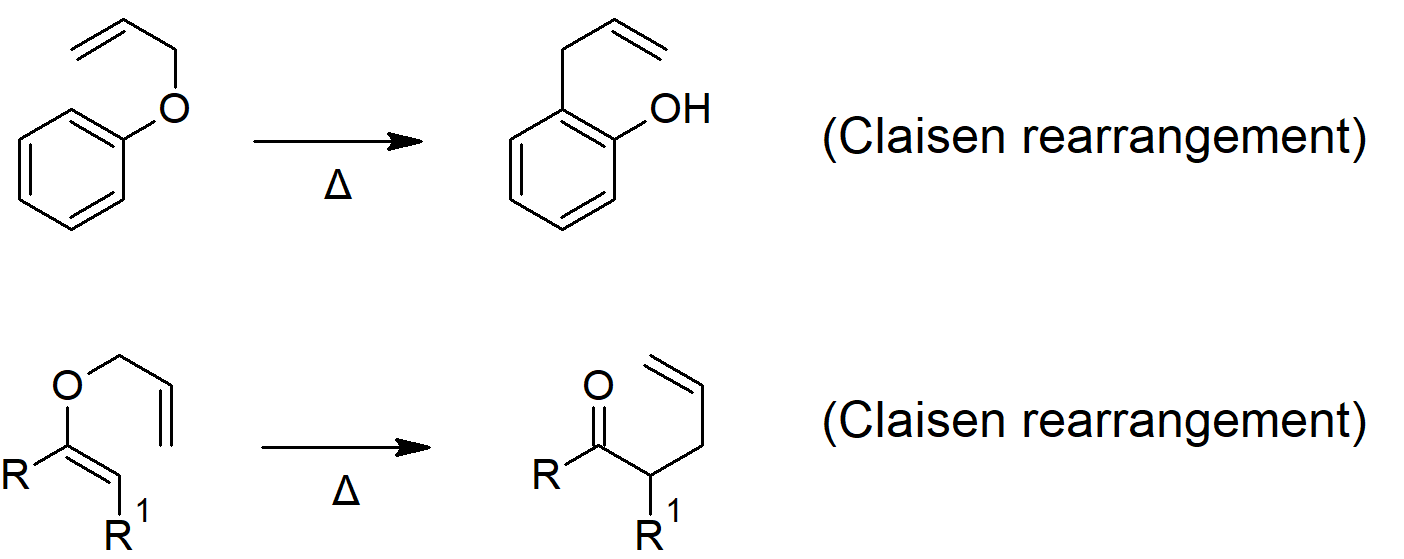
18.4 Reactions of Ethers: Claisen Rearrangement
- The Claisen rearrangement is a [3, 3] sigmatropic rearrangement reaction that converts aryl or enol ethers into carbonyl compounds (though the aromatic version rearranges into a phenol to re-establish aromaticity.
- Epoxides, also called oxiranes, have a three-membered ring structure with one oxygen and two carbon atoms.
- Epoxides can be formed from alkenes by reaction with peroxy acids (MCPBA for example).
- Epoxides can be formed from halohydrin molecules by reaction with a base, which causes an intramolecular Williamson ether synthesis.
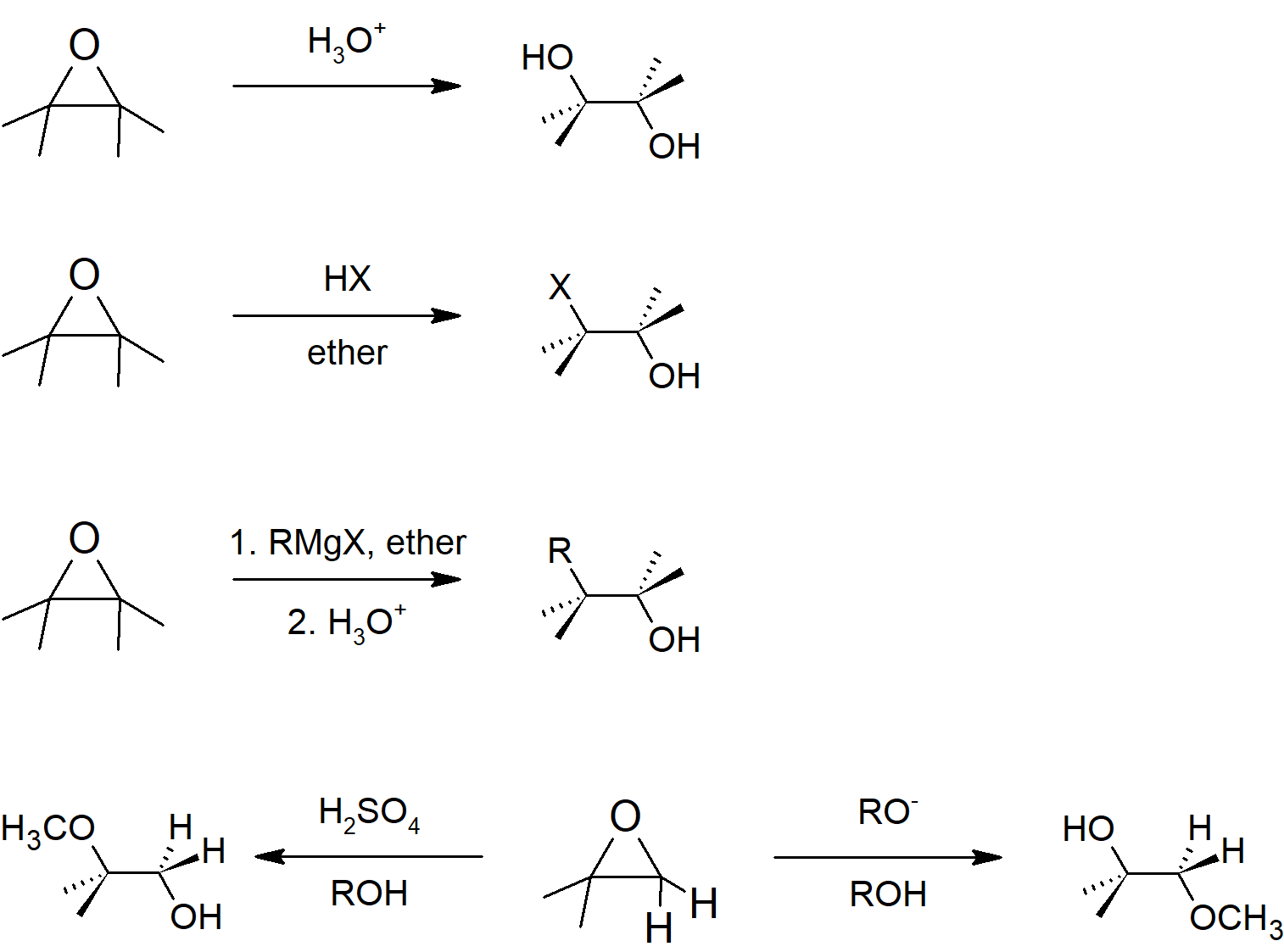
18.6 Reactions of Epoxides: Ring Opening
- When epoxides are ring opened under basic conditions, they follow SN2 mechanism leading to the nucleophile adding to the less substituted side of the epoxide.
- When epoxides are ring opened under acidic conditions, they follow SN1 mechanism leading to the nucleophile adding to the more substituted side of the epoxide.
- When epoxides are ring opened in aqueous reactions, the result is an anti-diol.
- Halo acids can be added to epoxides to form anti-halohydrins.
- Crown ethers are cyclic ethers containing several oxygen atoms.
- Crown ethers are named by the number of total atoms in the ring, followed by the word crown and finally the number of oxygen atoms (18-crown-6 for example).
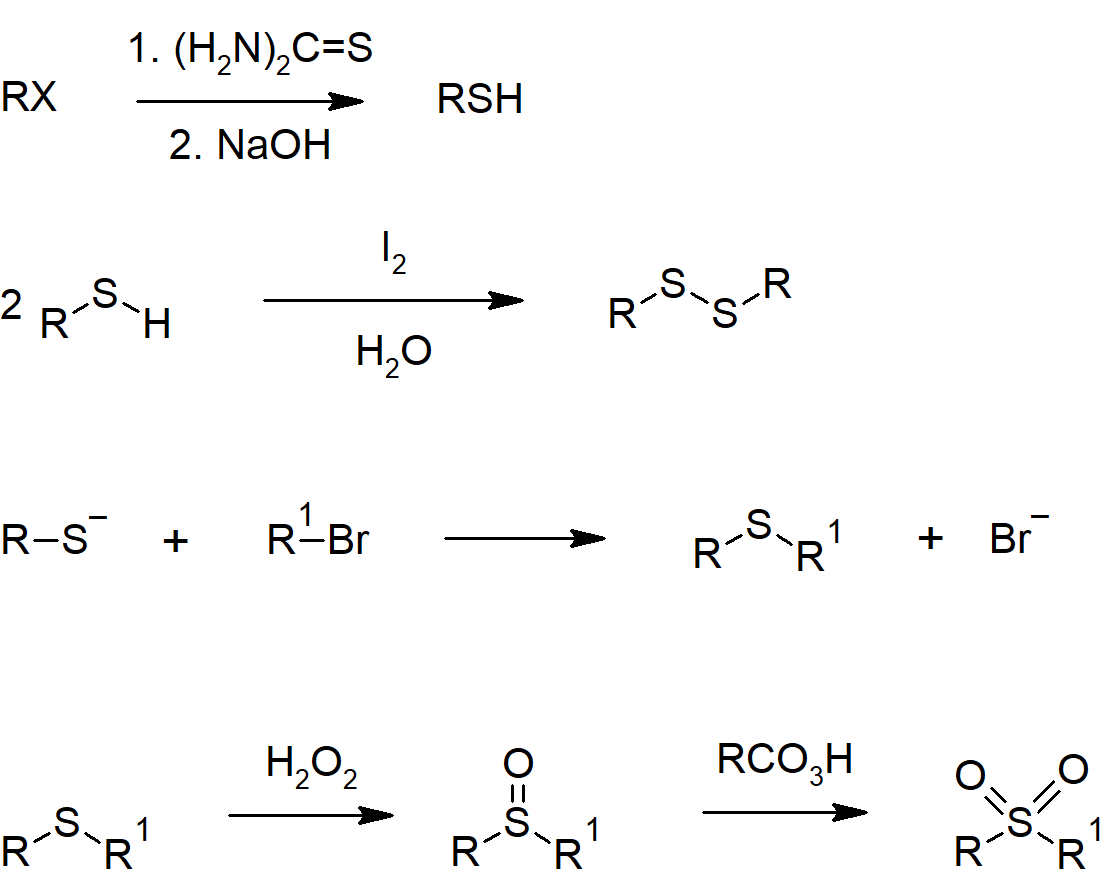
- Thiols can be prepared from alkyl halides through reaction with hydrosulfide ion (SH-) or through a more complicated series of reactions including thiourea.
- Thiols can be oxidized with mild oxidizing agents to form disulfides.
- Disulfide bridges link cysteine residues in protein structures.
- Sulfides are sulfur analogues of ether, though are much better nucleophiles with sulfur in place of oxygen.
- Ethers show standard C-H stretches and bends in IR along with a strong C-O stretch around 1000 cm-1.
- In 1H NMR, hydrogens on carbons adjacent to the oxygen typically appear between 3.4-4.5 ppm.
- Hydrogens on carbons of an epoxide ring typically appear between 2.5-3.5 ppm in 1H NMR.
18.10 Interchapter: A Preview of Carbonyl Chemistry
- Carbonyl groups are one of the most important features in organic chemistry and consist of a sp2 carbon double-bonded to oxygen.
- Carbonyl groups are present in ~10 different functional groups.
- Carbonyl groups are polarized with a partial positive charge on carbon and partial negative charge on oxygen. This makes the carbon atom an electrophile, while the oxygen can act as a nucleophile.
- Carbonyl groups can react through several mechanisms including nucleophilic addition and nucleophilic acyl substitution, alpha substitution and condensation.
Skills to Master
- Skill 18.1 Name ethers using common naming and IUPAC.
- Skill 18.2 Write reaction equations for preparation of ethers.
- Skill 18.3 Write mechanisms for reactions of ethers with strong halogen acids.
- Skill 18.4 Draw mechanisms for Cope and Claisen rearrangements.
- Skill 18.5 Draw mechanisms for ring-opening epoxides under acidic and basic conditions.
- Skill 18.6 Draw and name crown ethers.
- Skill 18.7 Explain how disulfide bridges contribute to protein structure.
- Skill 18.8 Give an example of S-adenosyl methionine activity in biological systems.
- Skill 18.9 Use IR and NMR spectra to identify ethers.
Summary of Reactions
Ether and Epoxide Preparation
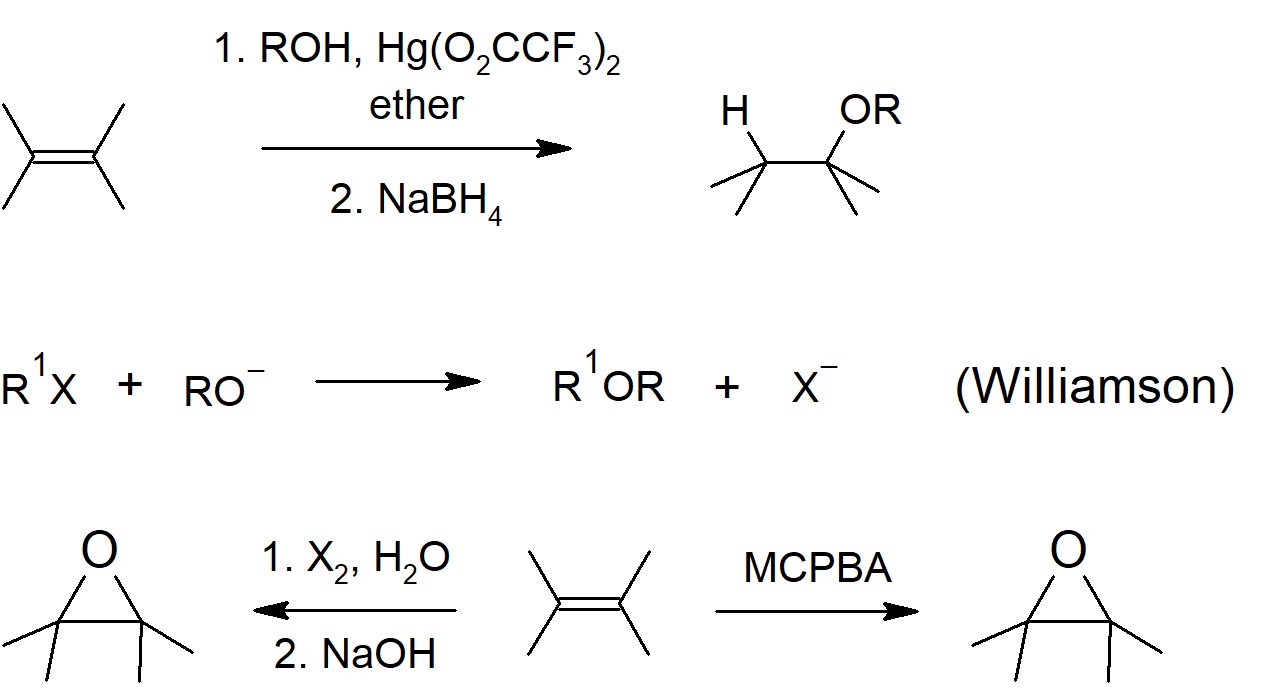
Ether Reactions
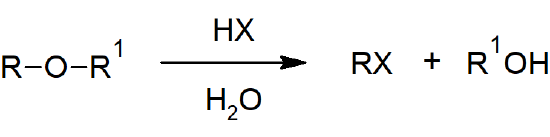
Epoxide Reactions
Sulfur Compound Reactions