23.8 Mixed Claisen Condensations
- Page ID
- 91021
Objectives
After completing this section, you should be able to
- write an equation to illustrate a mixed Claisen condensation.
- identify the structural features that should be present in the two esters if a mixed Claisen condensation is to be successful.
- determine whether a given pair of esters is likely to produce a good yield of a single product when subjected to a mixed Claisen condensation.
- identify the product formed when a given pair of esters is used in a mixed Claisen condensation.
- identify the esters that should be used to produce a given β‑keto ester by a mixed Claisen condensation.
- write an equation to illustrate the formation of a β‑diketone through a mixed Claisen‑type condensation between an ester and a ketone.
- identify the β‑diketone formed as the result of a mixed Claisen‑type condensation between a given ester and a given ketone.
- identify the reagents necessary to synthesize a given β‑diketone by a mixed Claisen‑type condensation between an ester and a ketone.
- write detailed mechanisms for mixed Claisen reactions and reactions that are related to the mixed Claisen reaction, including those in which both reacting moieties are present in the same compound.
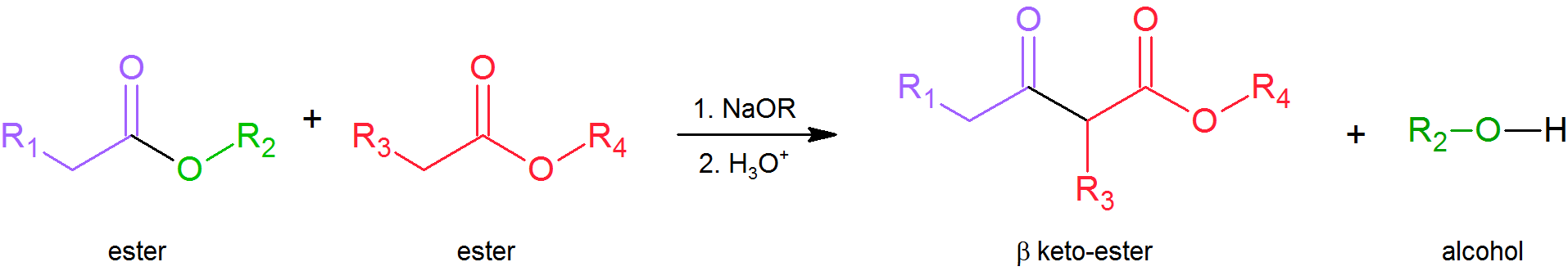
Just as we can react together two different aldehydes or ketones in a mixed aldol condensation, so can we react together two different esters in a mixed (or “crossed”) Claisen condensation. Again, by carefully selecting our substrates we can obtain a good yield of the desired product and minimize the number of possible by‑products. Note that even if we replace one of the esters with a ketone, the reaction is still referred to as Claisen condensation. The important thing to realize as you study these reactions is that they all take place by essentially the same mechanism—attack by an enolate anion on a carbonyl group.
Crossed Claisen Condensation
Claisen condensations between different ester reactants are called Crossed Claisen reactions. Crossed Claisen reactions in which both reactants can serve as donors and acceptors generally give complex mixtures. Because of this most Crossed Claisen reactions are usually not performed unless one reactant has no alpha hydrogens.
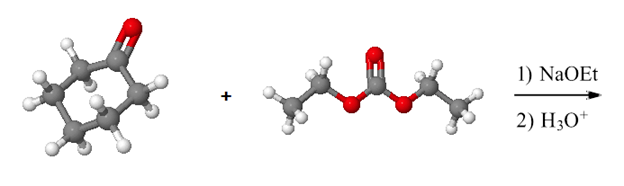
Show how the following molecule can be made using a Claisen-like condensation.
- Answer
-
Pathway 1
Solution 1
Pathway 2
Solution 2
Exercise
Draw the product of the following reactions:
a)
b)
- Answers
-
a)
b)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

