23.5 Mixed Aldol Reactions
- Page ID
- 91018
Objectives
After completing this section, you should be able to
- write an equation to illustrate a mixed aldol reaction.
- identify the structural features necessary to ensure that two carbonyl compounds will react together in a mixed aldol reaction to give a single product rather than a mixture of products.
- determine whether a given mixed aldol reaction is likely to produce a single product or a mixture of products.
- identify the product or products formed in a given mixed aldol reaction.
- identify the carbonyl compounds needed to produce a given enone or β‑hydroxy aldehyde or ketone by a mixed aldol reaction.
You should satisfy yourself that you understand how the four products shown in Example 23.5.2 arise from the condensation of 2‑propanone and 2‑phenylacetaldehyde.
The previous examples of aldol reactions and condensations used a common reactant as both the enolic donor and the electrophilic acceptor. The product in such cases is always a dimer of the reactant carbonyl compound. Aldol condensations between different carbonyl reactants are called crossed or mixed reactions, and under certain conditions such crossed aldol condensations can be effective.
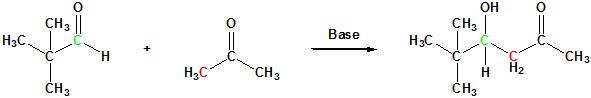
The success of these mixed aldol reactions is due to two factors. First, aldehydes are more reactive acceptor electrophiles than ketones, and formaldehyde is more reactive than other aldehydes. Second, aldehydes lacking alpha-hydrogens can only function as acceptor reactants, and this reduces the number of possible products by half. Mixed aldols in which both reactants can serve as donors and acceptors generally give complex mixtures of both dimeric (homo) aldols and crossed aldols. Because of this most mixed aldol reactions are usually not performed unless one reactant has no alpha hydrogens.
The following abbreviated formulas illustrate the possible products in such a case, red letters representing the acceptor component and blue the donor. If all the reactions occurred at the same rate, equal quantities of the four products would be obtained. Separation and purification of the components of such a mixture would be difficult.
ACH2CHO + BCH2CHO + NaOH  A–A + B–B + A–B + B–A
A–A + B–B + A–B + B–A
The aldol condensation of ketones with aryl aldehydes to form α,β-unsaturated derivatives is called the Claisen-Schmidt reaction.
Exercise
Show the reactants required to make the following using a mixed aldol condensation. Indicate those which would be likely to produce a mixture of products.
a)
b)
c)
- Answers
-
a)
b)
c) Because both reactants have α-hydrogens and neither are particularly acidic, the reaction probably will produce a mixture of condensation products.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

