22.3 Alpha Halogenation of Aldehydes and Ketones
- Page ID
- 91007
Objectives
After completing this section, you should be able to
- write an equation to illustrate the alpha halogenation of aldehydes and ketones.
- identify the product formed from the alpha halogenation of a given aldehyde or ketone.
- identify the carbonyl compound, the reagents, or both, needed to prepare a given α‑halogenated aldehyde or ketone.
- illustrate the importance of the alpha halogenation of carbonyl compounds as an intermediate step in the synthesis of α,β‑unsaturated aldehydes and ketones.
- write a detailed mechanism for the acid‑catalyzed halogenation of a ketone.
- describe the evidence provided by kinetic experiments supporting the suggestion that the acid‑catalyzed, alpha halogenation of ketones proceeds via the rate‑determining formation of an enol.
Note: α‑bromo ketones are a good starting material to generate α,β‑unsaturated ketones by dehydrobromination.
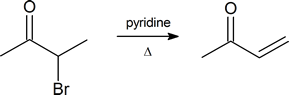
A carbonyl containing compound with α hydrogens can undergo a substitution reaction with halogens. This reaction comes about because of the tendency of carbonyl compounds to form enolates in basic condition and enols in acidic condition. In these cases even weak bases, such as the hydroxide anion, is sufficient enough to cause the reaction to occur because it is not necessary for a complete conversion to the enolate. For this reaction Cl2, Br2 or I2 can be used as the halogens.
General reaction
Acid Catalyzed Mechanism
Under acidic conditions the reaction occurs thought the formation of an enol which then reacts with the halogen.
1) Protonation of the carbonyl
2) Enol formation
3) SN2 attack
4) Deprotonation
Kinetic studies provide some evidence for the mechanism shown above. The rate law for the alpha-halogenation of a ketone can be given by:
rate = [ketone][H+]
The implication is that the rate determining step is dependent on the concentrations of the ketone and acid catalyst and therefore associated with the enol formation part of the mechanism. The halogen does not even appear in the rate law. Indeed, the overall rate is completely independent of the concentration of the halogen and suggests the halogenation step occurs rapidly.
Base Catalyzed Mechanism
Under basic conditions the enolate forms and then reacts with the halogen. Note! This is base promoted and not base catalyzed because an entire equivalent of base is required.
1) Enolate formation
2) SN2 attack
Overreaction during base promoted α halogenation
The fact that an electronegative halogen is placed on an α carbon means that the product of a base promoted α halogenation is actually more reactive than the starting material. The electron withdrawing effect of the halogen makes the α carbon even more acidic and therefor promotes further reaction. Because of this multiple halogenations can occur. This effect is exploited in the haloform reaction discussed later. If a monohalo product is required then acidic conditions are usually used.
Deuterium Exchange
Due to the acidic nature of α hydrogens they can be exchanged with deuterium by reaction with D2O (heavy water). The process is accelerated by the addition of an acid or base; an excess of D2O is required. The end result is the complete exchange of all α hydrogens with deuteriums.
Mechanism in basic conditions
1) Enolate Formation
2) Deuteration
Exercises
Please draw the products of the following reaction.
- Answer
-
Draw out the mechanism for the following reaction.
- Answer
-
How might you form 2-hepten-4-one from 4-heptanone?
- Answer
-
1) Br2, H3O+
2) Pyridine, Heat
Show the products of the following reactions:
- Answer
-
The following compound was reacted with D3O+. The only signals that could be found in the 1H NMR spectrum of the product were at 3.9 ppm (3H) and 6.6-6.9 ppm (4H). Please explain the results of the NMR.
- Answer
-
A deuterium exchange reaction occurred. All of the alpha-hydrogens in the molecule have been exchanged with deuterium. Because detueriums do not appear in a typical 1H NMR, only the remaining hydrogens appear.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

