9.5: Reduction of Alkynes
- Page ID
- 67217
Objectives
After completing this section, you should be able to
- write equations for the catalytic hydrogenation of alkynes to alkanes and cis alkenes.
- identify the reagent and catalyst required to produce a given alkane or cis alkene from a given alkyne.
- identify the product formed from the reaction of a given alkyne with hydrogen and a specified catalyst.
- identify the alkyne that must be used to produce a given alkane or cis alkene by catalytic hydrogenation.
- write the equation for the reduction of an alkyne with an alkali metal and liquid ammonia.
- predict the structure of the product formed when a given alkyne is reduced with an alkali metal and liquid ammonia.
- identify the alkyne that must be used to produce a given alkene by reduction with an alkali metal and ammonia.
Make certain that you can define, and use in context, the key terms below.
- anion radical
- Lindlar catalyst
The Lindlar catalyst allows a chemist to reduce a triple bond in the presence of a double bond.
Thus
but
Reactions between alkynes and catalysts are a common source of alkene formation. Because alkynes differ from alkenes on account of their two procurable π bonds, alkynes are more susceptible to additions. Aside from turning them into alkenes, these catalysts affect the arrangement of substituents on the newly formed alkene molecule. Depending on which catalyst is used, the catalysts cause anti- or syn-addition of hydrogens. Alkynes can readily undergo additions because of their availability of two π bonds.
Hydrogenation of an Alkyne
Alkynes can be fully hydrogenated into alkanes with the help of a platinum catalyst. However, the use of two other catalysts can be used to hydrogenate alkynes to alkanes. These catalysts are: Palladium dispersed on carbon (Pd/C) and finely dispersed nickel (Raney-Ni).
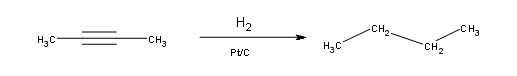
Hydrogenation of an Alkyne to a Cis-Alkene
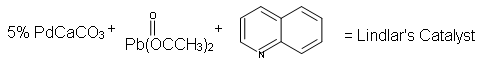
Because hydrogenation is an interruptible process involving a series of steps, hydrogenation can be stopped, using modified catalysts (e.g., Lindlar’s Catalyst) at the transitional alkene stage. Lindar’s catalyst has three components: Palladium-Calcium Carbonate, lead acetate and quinoline. The quinoline serves to prevent complete hydrogenation of the alkyne to an alkane. Lindlar’s Catalyst transforms an alkyne to a cis-alkene.
R-C≡C-R + H2 & Lindlar catalyst ——> cis R-CH=CH-R
Lindlar's Catalyst:
Like alkenes, alkynes readily undergo catalytic hydrogenation, either to cis or trans alkenes, or to alkanes, depending on the reaction employed.
The catalytic addition of hydrogen to 2-butyne provides heat of reaction data that reflect the relative thermodynamic stabilities of these hydrocarbons, as shown in the diagram to the right. From the heats of hydrogenation, shown in blue in units of kcal/mole, it would appear that alkynes are thermodynamically less stable than alkenes to a greater degree than alkenes are less stable than alkanes. The standard bond energies for carbon-carbon bonds confirm this conclusion. Thus, a double bond is stronger than a single bond, but not twice as strong. The difference ( 63 kcal/mole ) may be regarded as the strength of the π-bond component. Similarly, a triple bond is stronger than a double bond, but not 50% stronger. Here the difference ( 54 kcal/mole ) may be taken as the strength of the second π-bond. The 9 kcal/mole weakening of this second π-bond is reflected in the heat of hydrogenation numbers ( 36.7 - 28.3 = 8.4 ).
Since alkynes are thermodynamically less stable than alkenes, we might expect addition reactions of the former to be more exothermic and relatively faster than equivalent reactions of the latter. In the case of catalytic hydrogenation, the usual Pt and Pd hydrogenation catalysts are so effective in promoting addition of hydrogen to both double and triple carbon-carbon bonds that the alkene intermediate formed by hydrogen addition to an alkyne cannot be isolated. A less efficient catalyst, Lindlar's catalyst, prepared by deactivating (or poisoning) a conventional palladium catalyst by treating it with lead acetate and quinoline, permits alkynes to be converted to alkenes without further reduction to an alkane.
Hydrogenation of an Alkyne to a Trans-Alkene
Alkynes can be reduced to trans-alkenes with the use of sodium dissolved in an ammonia solvent. An Na radical donates an electron to one of the P bonds in a carbon-carbon triple bond. This forms an anion, which can be protonated by a hydrogen in an ammonia solvent. This prompts another Na radical to donate an electron to the second P orbital. Soon after this anion is also protonated by a hydrogen from the ammonia solvent, resulting in a trans-alkene.
R-C≡C-R + 2 Na in NH3 (liq) ——> trans R-CH=CH-R + 2 NaNH2
Mechanism
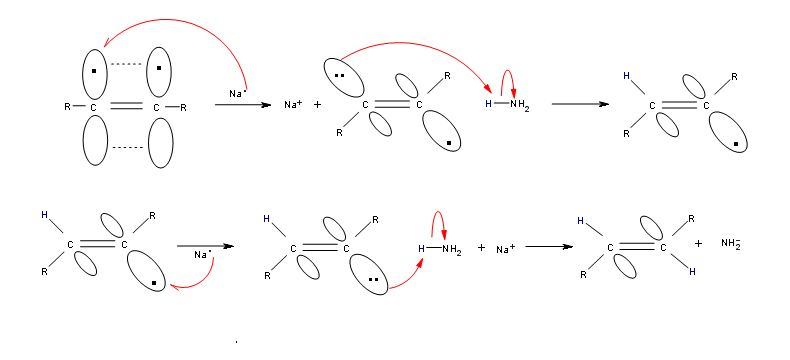
Overview of Reduction of Alkynes
Alkynes can undergo reductive hydrogenation reactions similar to alkenes. With the presence of two pi bonds within alkynes, the reduction reactions can be partial to form an alkene or complete to form an alkane. Since partial reduction of an alkyne produces an alkene, the stereochemistry provided by the reaction's mechanism determines whether a cis- or trans- alkene is formed. The three most significant alkyne reduction reactions are summarized below:
Exercise
Using any alkyne how would you prepare the following compounds: pentane, trans-4-methyl-2-pentene, cis-4-methyl-2-pentene.
- Answer
-
Contributors and Attributions
- Ravjot Takhar (UCD)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

