11.7: Elimination Reactions - Zaitsev's Rule
- Page ID
- 67268
Objective
After completing this section, you should be able to apply Zaitsev’s rule to predict the major product in a base-induced elimination of an unsymmetrical halide.
Make certain that you can define, and use in context, the key term below.
Zaitsev’s rule
Zaitsev's Rule can be used to predict the regiochemistry of elimination reactions.
Introduction
Zaitsev’s or Saytzev’s (anglicized spelling) rule is an empirical rule used to predict regioselectivity of 1,2-elimination reactions occurring via the E1 or E2 mechanisms. It states that in a regioselective E1 or E2 reaction the major product is the more stable alkene, (i.e., the alkene with the more highly substituted double bond). For example:
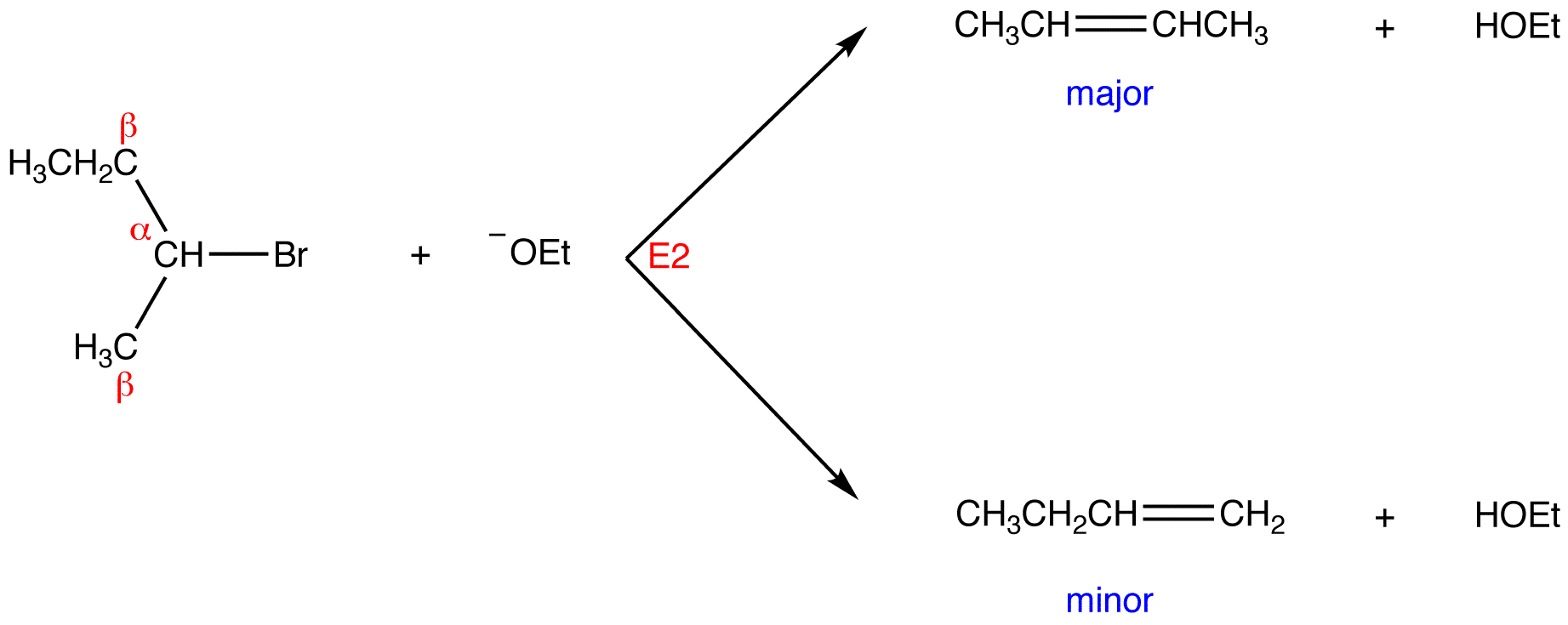
If two or more structurally distinct groups of beta-hydrogens are present in a given reactant, then several constitutionally isomeric alkenes may be formed by an E2 elimination. This situation is illustrated by the 2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.
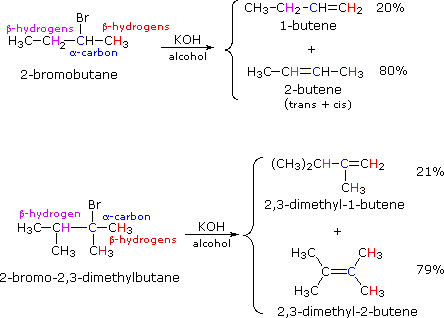
By using the strongly basic hydroxide nucleophile, we direct these reactions toward elimination. In both cases there are two different sets of beta-hydrogens available to the elimination reaction (these are colored red and magenta and the alpha carbon is blue). If the rate of each possible elimination was the same, we might expect the amounts of the isomeric elimination products to reflect the number of hydrogens that could participate in that reaction. For example, since there are three 1º-hydrogens (red) and two 2º-hydrogens (magenta) on beta-carbons in 2-bromobutane, statistics would suggest a 3:2 ratio of 1-butene and 2-butene in the products. This is not observed, and the latter predominates by 4:1. This departure from statistical expectation is even more pronounced in the second example, where there are six 1º-beta-hydrogens compared with one 3º-hydrogen. These results point to a strong regioselectivity favoring the more highly substituted product double bond, an empirical statement generally called the Zaitsev Rule.
Exercises
Ignoring the alkene stereochemistry show the elimination product(s) of the following compounds:
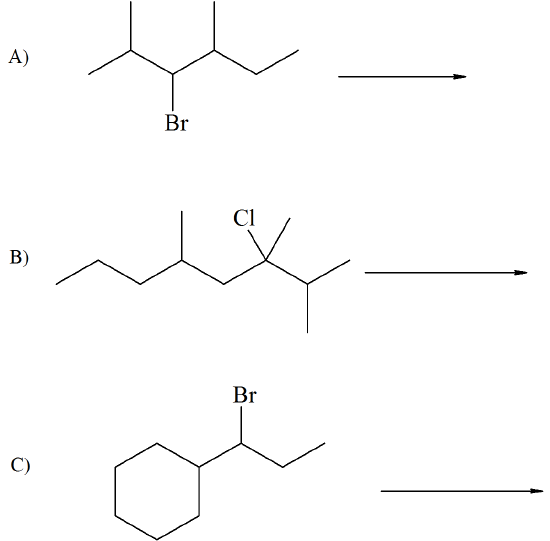
- Answer
-

Predict the major products of the following reactions.
a)
b)
c)
- Answer
-
a)
b)
c)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

