15.5: Aromatic Heterocycles- Pyridine and Pyrrole
- Page ID
- 67319
Objectives
After completing this section, you should be able to
- draw the structure of the common aromatic heterocycles pyridine and pyrrole.
- use the Hückel 4n + 2 rule to explain the aromaticity of each of pyridine and pyrrole.
- draw a diagram to show the orbitals involved in forming the conjugated six‑pi‑electron systems present in aromatic heterocycles such as pyridine, pyrrole, etc.
Make certain that you can define, and use in context, the key terms below.
- carbocycles
- heterocycles
Aromatic Heterocycles
Many unsaturated cyclic compounds have exceptional properties that we now consider characteristic of "aromatic" systems. The following cases are illustrative:
|
Compound |
Structural |
Reaction |
Thermodynamic |
|||
|
1,3-Cyclopentadiene |
Addition ( 0 ºC ) |
Slight |
||||
|
1,3,5-Cycloheptatriene |
Addition ( 0 ºC ) |
Slight |
||||
|
1,3,5,7-Cyclooctatetraene |
Addition ( 0 ºC ) |
Slight |
||||
|
Benzene |
Substitution |
Large |
||||
|
Pyridine |
Substitution |
Large |
||||
|
Furan |
Substitution ( 0 ºC ) |
Moderate |
||||
|
Pyrrole |
Substitution |
Moderate |
||||
Benzene is the archetypical aromatic compound. It is planar, bond angles=120º, all carbon atoms in the ring are sp2 hybridized, and the pi-orbitals are occupied by 6 electrons. The aromatic heterocycle pyridine is similar to benzene, and is often used as a weak base for scavenging protons. Furan and pyrrole have heterocyclic five-membered rings, in which the heteroatom has at least one pair of non-bonding valence shell electrons. By hybridizing this heteroatom to a sp2 state, a p-orbital occupied by a pair of electrons and oriented parallel to the carbon p-orbitals is created. The resulting planar ring meets the first requirement for aromaticity, and the π-system is occupied by 6 electrons, 4 from the two double bonds and 2 from the heteroatom, thus satisfying the Hückel Rule.
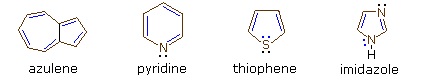
Four illustrative examples of aromatic compounds are shown above. The sp2 hybridized ring atoms are connected by brown bonds, the π-electron pairs and bonds that constitute the aromatic ring are colored blue. Electron pairs that are not part of the aromatic π-electron system are black. The first example is azulene, a blue-colored 10 π-electron aromatic hydrocarbon isomeric with naphthalene. The second and third compounds are heterocycles having aromatic properties. Pyridine has a benzene-like six-membered ring incorporating one nitrogen atom. The non-bonding electron pair on the nitrogen is not part of the aromatic π-electron sextet, and may bond to a proton or other electrophile without disrupting the aromatic system. In the case of thiophene, a sulfur analog of furan, one of the sulfur electron pairs (colored blue) participates in the aromatic ring π-electron conjugation. The last compound is imidazole, a heterocycle having two nitrogen atoms. Note that only one of the nitrogen non-bonding electron pairs is used for the aromatic π-electron sextet. The other electron pair (colored black) behaves similarly to the electron pair in pyridine.
Heterocycles - cyclic structures in which the ring atoms may include oxygen or nitrogen - can also be aromatic. Pyridine, for example, is an aromatic heterocycle. In the bonding picture for pyridine, the nitrogen is sp2-hybridized, with two of the three sp2 orbitals forming sigma overlaps with the sp2 orbitals of neighboring carbon atoms, and the third nitrogen sp2 orbital containing the lone pair. The unhybridized p orbital contains a single electron, which is part of the 6 pi-electron system delocalized around the ring.
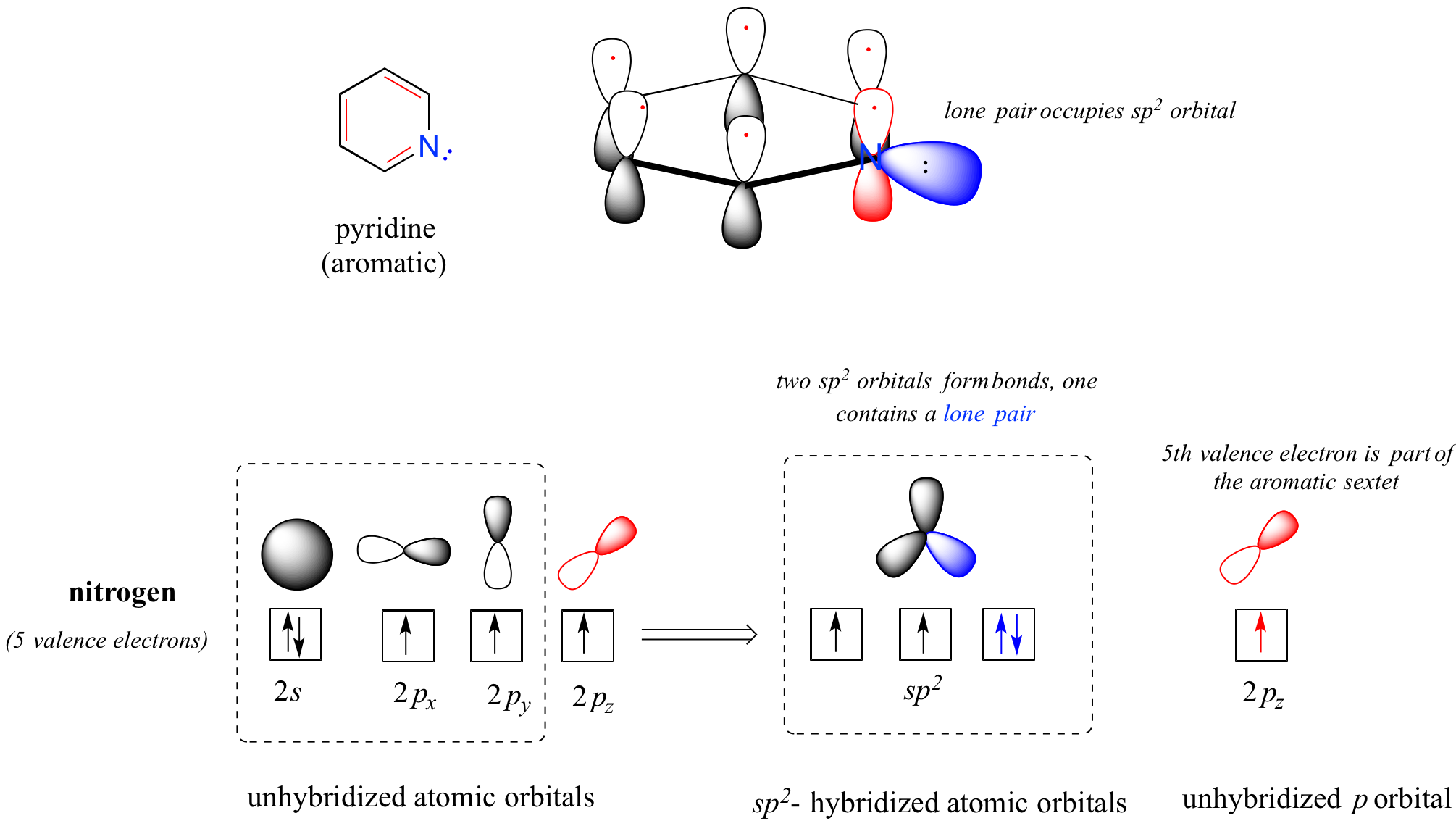
another image of orbitals in pyridine
Why do we not assume that the nitrogen in pyrrole is sp3-hybridized, like a normal secondary amine? The answer is simple: if it were, then pyrrole could not be aromatic, and thus it would not have the stability associated with aromaticity. In general, if a molecule or group can be aromatic, it will be, just as water will always flow downhill if there is a downhill pathway available.
Imidazole is another important example of an aromatic heterocycle found in biomolecules - the side chain of the amino acid histidine contains an imidazole ring.
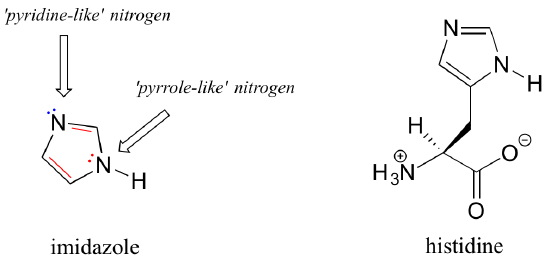
In imidazole, one nitrogen is 'pyrrole-like' (the lone pair contributes to the aromatic sextet) and one is 'pyridine-like' (the lone pair is located in an sp2 orbital, and is not part of the aromatic sextet).
Exercises
Draw the orbitals of thiophene to show that is aromatic.
- Answer
-
This drawing shows it has 6 electrons in the pi-orbital.
The following ring is called a thiazolium ring. Describe how it is aromatic.
- Answer
-
Similar to the last question, the drawing shows that there is only 6 electrons in the pi-system.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

